Sickle-cell disease
Not to be confused with Sick cell syndrome.
| Sickle-cell disease | |
|---|---|
| Classification and external resources | |

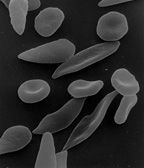
Figure (A) shows normal red blood cells flowing freely through
veins. The inset shows a cross section of a normal red blood cell with
normal haemoglobin. Figure B shows abnormal, sickled red blood cells log
jamming, sticking and accumulating at the branching point in a vein.
The inset image shows a cross-section of a sickle cell with long
polymerized HbS strands stretching and distorting the cell shape.
|
|
| ICD-10 | D57 |
| ICD-9 | 282.6 |
| OMIM | 603903 |
| DiseasesDB | 12069 |
| MedlinePlus | 000527 |
| eMedicine | med/2126 oph/490 ped/2096 emerg/26 emerg/406 |
| MeSH | C15.378.071.141.150.150 |
| GeneReviews | |
Life expectancy is shortened. In 1994, in the US, the average life expectancy of persons with this condition was estimated to be 42 years in males and 48 years in females,[1] but today, thanks to better management of the disease, patients can live into their 70s or beyond.[2]
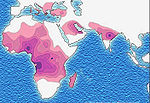
Sickle-cell disease occurs more commonly among people whose ancestors lived in tropical and sub-tropical sub-Saharan regions where malaria is or was common. Where malaria is common, carrying a single sickle-cell allele (sickle cell trait) confers a selective advantage—in other words, being a heterozygote is advantageous. Specifically, humans with one of the two alleles of sickle-cell disease show less severe symptoms when infected with malaria.[3]
Sickle-cell anaemia is a form of sickle-cell disease in which there is homozygosity for the mutation that causes HbS. Sickle-cell anaemia is also referred to as "HbSS", "SS disease", "haemoglobin S" or permutations of those names. In heterozygous people, that is, those who have only one sickle gene and one normal adult haemoglobin gene, the condition is referred to as "HbAS" or "sickle cell trait". Other, rarer forms of sickle-cell disease are compound heterozygous states in which the person has only one copy of the mutation that causes HbS and one copy of another abnormal haemoglobin allele. They include sickle-haemoglobin C disease (HbSC), sickle beta-plus-thalassaemia (HbS/β+) and sickle beta-zero-thalassaemia (HbS/β0).
The term disease is applied because the inherited abnormality causes a pathological condition that can lead to death and severe complications. Not all inherited variants of haemoglobin are detrimental, a concept known as genetic polymorphism.
Contents
Signs and symptoms
Sickle-cell disease may lead to various acute and chronic complications, several of which have a high mortality rate.[4]Sickle cell crisis
The terms "sickle cell crisis" or "sickling crisis" may be used to describe several independent acute conditions occurring in patients with sickle cell disease. Sickle cell disease results in anemia and crises that could be of many types including the vaso-occlusive crisis, aplastic crisis, sequestration crisis, haemolytic crisis and others. Most episodes of sickle cell crises last between five and seven days.[5] "Although infection, dehydration, and acidosis (all of which favor sickling) can act as triggers, in most instances no predisposing cause is identified."[6]Vaso-occlusive crisis
The vaso-occlusive crisis is caused by sickle-shaped red blood cells that obstruct capillaries and restrict blood flow to an organ resulting in ischaemia, pain, necrosis and often organ damage. The frequency, severity, and duration of these crises vary considerably. Painful crises are treated with hydration, analgesics, and blood transfusion; pain management requires opioid administration at regular intervals until the crisis has settled. For milder crises, a subgroup of patients manage on NSAIDs (such as diclofenac or naproxen). For more severe crises, most patients require inpatient management for intravenous opioids; patient-controlled analgesia (PCA) devices are commonly used in this setting. Vaso-occlusive crisis involving organs such as the penis [7] or lungs are considered an emergency and treated with red-blood cell transfusions. Diphenhydramine is sometimes effective for the itching associated with the opioid use. Incentive spirometry, a technique to encourage deep breathing to minimise the development of atelectasis, is recommended.[8]Splenic sequestration crisis
Because of its narrow vessels and function in clearing defective red blood cells, the spleen is frequently affected.[9] It is usually infarcted before the end of childhood in individuals suffering from sickle-cell anemia. This autosplenectomy increases the risk of infection from encapsulated organisms;[10][11] preventive antibiotics and vaccinations are recommended for those with such asplenia.Splenic sequestration crises are acute, painful enlargements of the spleen, caused by intrasplenic trapping of red cells and resulting in a precipitous fall in hemoglobin levels with the potential for hypovolemic shock. Sequestration crises are considered an emergency. If not treated, patients may die within 1–2 hours due to circulatory failure. Management is supportive, sometimes with blood transfusion. These crises are transient, they continue for 3–4 hours and may last for one day.[12]
Acute chest syndrome ACS
Acute chest syndrome (ACS) is defined by new pulmonary infiltrate with a manifestation of pulmonary symptoms like tachypnea and dyspnea.[13] It is the second most common complication and it accounts for about 25% of death in patients with SCD, majority of cases present with vaso occlusive crises then they develop ACS.[13][14] Nevertheless, in Dessap et al., 2007 study they reported that about 80% of patients has vaso occlusive crises during ACS.Aplastic crisis
Aplastic crises are acute worsenings of the patient's baseline anaemia, producing pallor, tachycardia, and fatigue. This crisis is normally triggered by parvovirus B19, which directly affects production of red blood cells by invading the red cell precursors and multiplying in them and destroying them.[15] Parvovirus infection nearly completely prevents red blood cell production for two to three days. In normal individuals, this is of little consequence, but the shortened red cell life of sickle-cell patients results in an abrupt, life-threatening situation. Reticulocyte counts drop dramatically during the disease (causing reticulocytopenia), and the rapid turnover of red cells leads to the drop in haemoglobin. This crisis takes 4 days to one week to disappear. Most patients can be managed supportively; some need blood transfusion.[16]Haemolytic crisis
Haemolytic crises are acute accelerated drops in haemoglobin level. The red blood cells break down at a faster rate. This is particularly common in patients with co-existent G6PD deficiency.[17] Management is supportive, sometimes with blood transfusions.[8]Other
One of the earliest clinical manifestations is dactylitis, presenting as early as six months of age, and may occur in children with sickle trait.[18] The crisis can last up to a month.[19] Another recognised type of sickle crisis is the acute chest syndrome, a condition characterised by fever, chest pain, difficulty breathing, and pulmonary infiltrate on a chest X-ray. Given that pneumonia and sickling in the lung can both produce these symptoms, the patient is treated for both conditions.[20] It can be triggered by painful crisis, respiratory infection, bone-marrow embolisation, or possibly by atelectasis, opiate administration, or surgery.HETEROZYGOUS:
The heterozygous form (sickle cell trait) is almost always asymptomatic, and the only usual significant manifestation is the renal concentrating defect presenting with isosthenuria.
Pathophysiology
The loss of red blood cell elasticity is central to the pathophysiology of sickle-cell disease. Normal red blood cells are quite elastic, which allows the cells to deform to pass through capillaries. In sickle-cell disease, low-oxygen tension promotes red blood cell sickling and repeated episodes of sickling damage the cell membrane and decrease the cell's elasticity. These cells fail to return to normal shape when normal oxygen tension is restored. As a consequence, these rigid blood cells are unable to deform as they pass through narrow capillaries, leading to vessel occlusion and ischaemia.
The actual anaemia of the illness is caused by haemolysis, the destruction of the red cells, because of their misshape. Although the bone marrow attempts to compensate by creating new red cells, it does not match the rate of destruction.[34] Healthy red blood cells typically live 90–120 days, but sickle cells only survive 10–20 days.[35]
Genetics
Normally, humans have Haemoglobin A, which consists of two alpha and two beta chains, Haemoglobin A2, which consists of two alpha and two delta chains and Haemoglobin F, consisting of two alpha and two gamma chains in their bodies. Of these, Haemoglobin A makes up around 96-97% of the normal haemoglobin in humans.Sickle-cell gene mutation probably arose spontaneously in different geographic areas, as suggested by restriction endonuclease analysis. These variants are known as Cameroon, Senegal, Benin, Bantu and Saudi-Asian. Their clinical importance springs from the fact that some of them are associated with higher HbF levels, e.g., Senegal and Saudi-Asian variants, and tend to have milder disease.[36]
In people heterozygous for HgbS (carriers of sickling haemoglobin), the polymerisation problems are minor, because the normal allele is able to produce over 50% of the haemoglobin. In people homozygous for HgbS, the presence of long-chain polymers of HbS distort the shape of the red blood cell from a smooth doughnut-like shape to ragged and full of spikes, making it fragile and susceptible to breaking within capillaries. Carriers have symptoms only if they are deprived of oxygen (for example, while climbing a mountain) or while severely dehydrated. The sickle-cell disease occurs when the sixth amino acid, glutamic acid, is replaced by valine to change its structure and function; as such, sickle cell anemia is also known as E6V. Valine is hydrophobic, causing the haemoglobin to collapse in on itself occasionally. The structure is not changed otherwise. When enough haemoglobin collapses in on itself the red blood cells become sickle-shaped.
The allele responsible for sickle-cell anaemia can be found on the short arm of chromosome 11. A person that receives the defective gene from both father and mother develops the disease; a person that receives one defective and one healthy allele remains healthy, but can pass on the disease and is known as a carrier. If two parents who are carriers have a child, there is a 1-in-4 chance of their child developing the disease and a 1-in-2 chance of their child being just a carrier. Heterozygotes are still able to contract malaria, but their symptoms are generally less severe.[37]
Due to the adaptive advantage of the heterozygote, the disease is still prevalent, especially among people with recent ancestry in malaria-stricken areas, such as Africa, the Mediterranean, India and the Middle East.[38] Malaria was historically endemic to southern Europe, but it was declared eradicated in the mid-20th century, with the exception of rare sporadic cases.[39]
The malaria parasite has a complex life cycle and spends part of it in red blood cells. In a carrier, the presence of the malaria parasite causes the red blood cells with defective haemoglobin to rupture prematurely, making the plasmodium unable to reproduce. Further, the polymerization of Hb affects the ability of the parasite to digest Hb in the first place. Therefore, in areas where malaria is a problem, people's chances of survival actually increase if they carry sickle-cell trait (selection for the heterozygote).
In the USA, where there is no endemic malaria, the prevalence of sickle-cell anaemia among blacks is lower (about 0.25%) than in West Africa (about 4.0%) and is falling. Without endemic malaria, the sickle cell mutation is purely disadvantageous and will tend to be selected out of the affected population via natural selection. However, the African American community of the USA is known to be the result of significant admixture between several African and non-African ethnic groups, and also represents the descendants of survivors of the slavery and the slave trade. Thus, a lower degree of endogamy and, particularly, abnormally high health-selective pressure through slavery may be the most plausible explanations for the lower prevalence of sickle-cell anaemia (and, possibly, other genetic diseases) among African-Americans compared to Sub-Saharan Africans. Another factor limiting the spread of sickle-cell genes in North America is the absence of cultural proclivities to polygamy, which allows affected males to continue to seek unaffected children with multiple partners.[40]
Inheritance
Sickle-cell conditions have an autosomal recessive pattern of inheritance from parents.[41] The types of haemoglobin a person makes in the red blood cells depend on what haemoglobin genes are inherited from her or his parents. If one parent has sickle-cell anaemia (SS) and the other has sickle-cell trait then there is a 50% chance of a child's having sickle-cell disease and a 50% chance of a child's having sickle-cell trait. When both parents have sickle-cell trait a child has a 25% chance of sickle-cell disease, 25% will not carry any sickle cell alleles, and 50% will have the heterozygous condition, as shown in the diagram.Diagnosis
In HbSS, the full blood count reveals haemoglobin levels in the range of 6–8 g/dL with a high reticulocyte count (as the bone marrow compensates for the destruction of sickle cells by producing more red blood cells). In other forms of sickle-cell disease, Hb levels tend to be higher. A blood film may show features of hyposplenism (target cells and Howell-Jolly bodies).Sickling of the red blood cells, on a blood film, can be induced by the addition of sodium metabisulfite. The presence of sickle haemoglobin can also be demonstrated with the "sickle solubility test". A mixture of haemoglobin S (Hb S) in a reducing solution (such as sodium dithionite) gives a turbid appearance, whereas normal Hb gives a clear solution.
Abnormal haemoglobin forms can be detected on haemoglobin electrophoresis, a form of gel electrophoresis on which the various types of haemoglobin move at varying speeds. Sickle-cell haemoglobin (HgbS) and haemoglobin C with sickling (HgbSC)—the two most common forms—can be identified from there. The diagnosis can be confirmed with high-performance liquid chromatography (HPLC). Genetic testing is rarely performed, as other investigations are highly specific for HbS and HbC.[42]
An acute sickle-cell crisis is often precipitated by infection. Therefore, a urinalysis to detect an occult urinary tract infection, and chest X-ray to look for occult pneumonia should be routinely performed.[43]
People who are known carriers of the disease often undergo genetic counseling before they have a child. A test to see if an unborn child has the disease takes either a blood sample from the fetus or a sample of amniotic fluid. Since taking a blood sample from a fetus has greater risks, the latter test is usually used. Neonatal screening provides not only a method of early detection for individuals with sickle cell disease, but also allows for identification of the groups of people that carry the sickle cell trait.[44]
After the mutation responsible for this disease was discovered in 1979, the U.S. Air Force required black applicants to test for the mutation. It dismissed 143 applicants because they were carriers, even though none of them had the condition. It eventually withdrew the requirement, but only after a trainee filed a lawsuit.[45]
Management
Folic acid and penicillin
Children born with sickle-cell disease will undergo close observation by the pediatrician and will require management by a haematologist to assure they remain healthy. These patients will take a 1 mg dose of folic acid daily for life. From birth to five years of age, they will also have to take penicillin daily due to the immature immune system that makes them more prone to early childhood illnesses.Malaria chemoprophylaxis
The protective effect of sickle cell trait does not apply to people with sickle cell disease; in fact, they are uniquely vulnerable to malaria, since the most common cause of painful crises in malarial countries is infection with malaria. It has therefore been recommended that people with sickle cell disease living in malarial countries should receive anti-malarial chemoprophylaxis for life.[46]Vaso-occlusive crises
Most people with sickle-cell disease have intensely painful episodes called vaso-occlusive crises. The frequency, severity, and duration of these crises, however, vary tremendously. Painful crises are treated symptomatically with analgesics; pain management requires opioid administration at regular intervals until the crisis has settled. For milder crises, a subgroup of patients manage on NSAIDs (such as diclofenac or naproxen). For more severe crises, most patients require inpatient management for intravenous opioids; patient-controlled analgesia (PCA) devices are commonly used in this setting. Diphenhydramine is also an effective agent that is frequently prescribed by doctors in order to help control any itching associated with the use of opioids.Acute chest crisis
Management is similar to vaso-occlusive crisis, with the addition of antibiotics (usually a quinolone or macrolide, since cell wall-deficient ["atypical"] bacteria are thought to contribute to the syndrome),[47] oxygen supplementation for hypoxia, and close observation. Should the pulmonary infiltrate worsen or the oxygen requirements increase, simple blood transfusion or exchange transfusion is indicated. The latter involves the exchange of a significant portion of the patients red cell mass for normal red cells, which decreases the percent of haemoglobin S in the patient's blood.Hydroxyurea
The first approved drug for the causative treatment of sickle-cell anaemia, hydroxyurea, was shown to decrease the number and severity of attacks in a study in 1995 (Charache et al.)[48] and shown to possibly increase survival time in a study in 2003 (Steinberg et al.).[49] This is achieved, in part, by reactivating fetal haemoglobin production in place of the haemoglobin S that causes sickle-cell anaemia. Hydroxyurea had previously been used as a chemotherapy agent, and there is some concern that long-term use may be harmful, but this risk has been shown to be either absent or very small and it is likely that the benefits outweigh the risks.[50]Transfusion therapy
Blood transfusions are often used in the management of sickle cell disease in acute cases and to prevent complications by decreasing the number of red blood cells (RBC) that can sickle by adding normal red blood cells.[51] In children prophylactic chronic red blood cell (RBC) transfusion therapy has been shown to be efficacious to a certain extent in reducing the risk of first stroke or silent stroke when transcranial Doppler (TCD) ultrasonography shows abnormal increased cerebral blood flow velocities. In those who have sustained a prior stroke event it also reduces the risk of recurrent stroke and additional silent strokes.[52][53]Bone marrow transplants
Bone marrow transplants have proven to be effective in children. Bone marrow transplants are the only known cure for SCD.[54] However, bone marrow transplants are difficult to obtain because of the specific HLA typing necessary. Ideally, a twin family member (syngeneic) or close relative (allogeneic) would donate the bone marrow necessary for transplantation.Prognosis
About 90% of patients survive to age 20, and close to 50% survive beyond the fifth decade.[55] In 2001, according to one study, the estimated mean survival for sickle cell patients was 53 years old for men and 58 years old for women with homozygous SCD.[56]Epidemiology
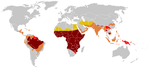
The highest frequency of sickle cell disease is found in tropical regions, particularly sub-Saharan Africa, India and the Middle-East.[57] Migration of substantial populations from these high prevalence areas to low prevalence countries in Europe has dramatically increased in recent decades and in some European countries sickle cell disease has now overtaken more familiar genetic conditions such as haemophilia and cystic fibrosis.[58] In 2010, there were about 29,000 deaths attributed to sickle cell disease globally.[59]Africa
Three quarters of sickle-cell cases occur in Africa. A recent WHO report estimated that around 2% of newborns in Nigeria were affected by sickle cell anaemia, giving a total of 150,000 affected children born every year in Nigeria alone. The carrier frequency ranges between 10% and 40% across equatorial Africa, decreasing to 1–2% on the north African coast and <1% in South Africa.[60]United States
The prevalence of the disease in the United States is approximately 1 in 5,000, mostly affecting Americans of Sub-Saharan African descent, according to the National Institutes of Health.[61] In the United States, about 1 out of 500 African-American children and 1 in every 36,000 Hispanic-American children born will have sickle-cell anaemia.[62] It is estimated that Sickle Cell Disease (SCD) affects 90,000 Americans.[63] Most infants with SCD born in the United States are now identified by routine neonatal screening. Forty-four states along with the District of Columbia, Puerto Rico and the Virgin Islands currently provide universal neonatal screening for SCD.[64][65] Sickle Cell trait occurs among about 1:12 African-Americans and 1:100 Hispanic-Americans.[66] It is estimated that 2.5 million Americans are heterozygous carriers for the sickle cell trait.[67]France
As a result of population growth in African-Caribbean regions of overseas France and immigration from North and sub-Saharan Africa to mainland France, sickle cell disease has become a major health problem in France.[68] SCD has become the most common genetic disease in the country, with an overall birth prevalence of 1/2,415 in mainland France, ahead of phenylketonuria (1/10,862), congenital hypothyroidism (1/3,132), congenital adrenal hyperplasia (1/19,008) and cystic fibrosis (1/5,014) for the same reference period. In 2010, 31.5% of all newborns in mainland France (253,466 out of 805,958) were screened for SCD (this percentage was 19% in 2000). 341 newborns with SCD and 8,744 heterozygous carriers were found representing 1.1% of all newborns in mainland France. The Paris metropolitan district (Île-de-France) is the region that accounts for the largest number of newborns screened for SCD (60% in 2010). The second largest number of at-risk is in Provence-Alpes-Côte d'Azur at nearly 43.2% and the lowest number is in Brittany at 5.5%.[69][70]United Kingdom
In the United Kingdom, all babies receive a blood test to screen for this condition.[71]Middle East
In Saudi Arabia about 4.2% of the population carry the sickle-cell trait and 0.26% have sickle cell disease. The highest prevalence is in the Eastern province where approximately 17% of the population carry the gene and 1.2% have sickle cell disease.[72] In 2005 in Saudi Arabia a mandatory pre-marital test including HB electrophoresis was launched and aimed to decrease the incidence of SCD and thalassemia.[73]India
Sickle cell disease is common in many parts of India, where the prevalence has ranged from 9.4 to 22.2% in endemic areas.[74]Caribbean Islands
In Jamaica, 10% of the population carries the sickle cell gene, making it the most prevalent genetic disorder in the country.[75]History
| This section may require cleanup to meet Wikipedia's quality standards. The specific problem is: merge from Genetic resistance to malaria#Sickle cell History; needs integration with existing text. (March 2014) |
In 1910 a Chicago physician, James B. Herrick, reported the presence of sickle cells in the blood of an anaemic dental student, Walter Clement Noel.[77] These cells had first been observed by his intern Ernest Irons while they were treating Noel in 1904.
An association with pigmented gall stones was noted in 1911 by Washborn. A genetic basis for this disease was proposed in 1915 by Cook and Meyer. The disease was named sickle cell anaemia in 1922 by Verne Mason after several additional cases were reported. All the known cases had been reported in blacks and he concluded that this disease was confined to those of black African descent. The heterozygous condition was independently recognised in 1923 by Huck and Syndestrickler. Syndestrickler also was the first to note the splenic atrophy that occurs in this condition. It was recognised as a Mendelian autosomal characteristic by Taliaffero and Huck also in 1923.[78] A predisposition to pneumonia was noted in 1924 by Graham. The concept of progressive splenic atrophy was proposed by Hahn and Gilespie in 1927. Pneumococcal meningitis in this condition was first reported in 1928 by Wollstein and Kriedel but it was not until 1966 that the association between splenic atrophy and infection was made by Robinson and Watson.
In 1927 Vernon Hahn and Elizabeth Biermann Gillespie showed that sickling of the red cells was related to low oxygen.[79] In some individuals this change occurs at partial pressures of O
2 prevalent in the body, and produces anemia and other disorders, termed sickle-cell disease. In other persons sickling occurs only at very low O
2 partial pressures; these are asymptomatic sickle-cell trait carriers.
The association with kidney and lung infarcts was noted in 1931 by Yater and Mollari and Baird in 1934 respectively. The term sickle cell trait was coined by Samuel Diggs in Memphis in 1933 to distinguish heterozygotes from those with sickle cell anaemia. Diggs also reported the association with splenic fibrosis in 1935. The pathological mechanism of vaso-occlusion was proposed by Ham and Castle in 1940.
In 1946, E A Beet, a British medical officer stationed in Southern Rhodesia (Zimbabwe), observed that blood from malaria patients who had sickle cell trait had fewer malarial parasites than blood from patients without the trait and suggested that this might be a protective feature. In 1947 Beet published that the incidence of enlarged spleens in sickle cell patients was much lower than in non sickle cell and suggested that this was due to recurrent thromboses which resulted in fibrosis and shrinkage of the spleen. In 1949 Lehmann and Raper published a map of Uganda and showed that the presence of sickle cell anaemia correlated with the presence of malaria.[80] In 1950 Singer et al. noted the abrupt cessation of marrow activity that may occur and coined the term aplastic crisis. The role of parvovirus in aetiology of this condition was not recognised until 1981. P. Brain also while working in Northern Rhodesia confirmed the lower incidence of splenomegaly and suggested that while homozygotes for the sickle cell gene suffered from several problems heterozygotes might be protected against malaria.[81]
The modern phase of research on this disorder was initiated by the famous chemist Linus Pauling in 1949. Pauling postulated that the hemoglobin (Hb) in sickle-cell disease is abnormal; when deoxygenated it polymerizes into long, thin, helical rods that distort the red cell into a sickle shape. In his laboratory, electrophoretic studies showed that sickle-cell Hb (S) is indeed abnormal, having at physiological pH a lower negative charge than normal adult human Hb (A).[82] In sickle-cell trait carriers there is a nearly equal amount of HbA and HbS, whereas in persons with sickle-cell disease nearly all the Hb is of the S type, apart from a small amount of fetal Hb. These observations showed that most patients with sickle-cell disease are homozygous for the gene encoding HbS, while trait carriers are heterozygous for this gene. Persons inheriting a sickle-cell gene and another mutant at the same locus, e.g. a thalassemia gene, can also have a variant form of sickle-cell disease. Pauling also introduced the term "molecular disease", which, together with molecular medicine, has become widely used.
The next major advance was the discovery by Vernon Ingram in 1959 that HbS differs from HbA by only a single amino-acid substitution in the β-polypeptide chain (β6Glu → Val).[83] It was later established that this results from a substitution of thymine for adenine in the DNA codon (GAG → GTG). This was the first example in any species of the effects of a mutation on a protein.
This collection of clinical findings was unknown until the explanation of the sickle cells in 1910 by a Chicago cardiologist and professor of medicine James B. Herrick (1861–1954), whose intern Ernest Edward Irons (1877–1959) found "peculiar elongated and sickle-shaped" cells in the blood of Walter Clement Noel, a 20-year-old first-year dental student from Grenada, after Noel was admitted to the Chicago Presbyterian Hospital in December 1904 suffering from anaemia.[84]
Noel was readmitted several times over the next three years for "muscular rheumatism" and "bilious attacks". Noel completed his studies and returned to the capital of Grenada (St. George's) to practice dentistry. He died of pneumonia in 1916 and is buried in the Catholic cemetery at Sauteurs in the north of Grenada.[85] Herrick's published account included illustrations, but the earliest available slide showing sickle cells is that of a 1918 autopsy from a soldier with sickle trait, initially reviewed only 92 years later.[86]
The disease was named "sickle-cell anemia" by Verne Mason in 1922, then a medical resident at Johns Hopkins Hospital.[87] However, some elements of the disease had been recognized earlier: A paper in the Southern Journal of Medical Pharmacology in 1846 described the absence of a spleen in the autopsy of a runaway slave. The African medical literature reported this condition in the 1870s, when it was known locally as ogbanjes ("children who come and go") because of the very high infant mortality rate caused by this condition. A history of the condition tracked reports back to 1670 in one Ghanaian family.[88]
Linus Pauling and colleagues were the first, in 1949, to demonstrate that sickle-cell disease occurs as a result of an abnormality in the haemoglobin molecule. This was the first time a genetic disease was linked to a mutation of a specific protein, a milestone in the history of molecular biology, and it was published in their paper "Sickle Cell Anemia, a Molecular Disease".


















No comments:
Post a Comment