- Male Infertility
- Female Infertility
- Azoospermia
- Oligospermia
- Spermatogenesis
- Fallopian Tube
- Fallopian Tube Obstruction (blockage)
- Uterine fibroids
- Leukorrhea
- Ovarian cyst
- Hrmone Imbalance
- Chromosome
Male infertility
| Male infertility | |
|---|---|
Male infertility refers to a male's inability to cause pregnancy in a fertile female. In humans it accounts for 40-50% of infertility.[1][2][3]Male infertility is commonly due to deficiencies in the semen, and semen quality is used as a surrogate measure of male fecundity.[4]
Causes
Factors relating to male infertility include:[5]
Pre-testicular causes
Pre-testicular factors refer to conditions that impede adequate support of the testes and include situations of poor hormonal support and poor general health including:
- Hypogonadotropic hypogonadism due to various causes
- Obesity increases the risk of hypogonadotropic hypogonadism.[6] Animal models indicate that obesity causes leptin insensitivity in the hypothalamus, leading to decreasedKiss1 expression, which, in turn, alters the release of gonadotropin-releasing hormone (GnRH).[6]
- Drugs, alcohol
- Strenuous riding (bicycle riding,[7] horseback riding)
- Medications, including those that affect spermatogenesis such as chemotherapy, anabolic steroids, cimetidine, spironolactone; those that decrease FSH levels such asphenytoin; those that decrease sperm motility such as sulfasalazine and nitrofurantoin
- Genetic abnormalities such as a Robertsonian translocation
Tobacco smoking
Male smokers also have approximately 30% higher odds of infertility.[8] There is increasing evidence that the harmful products of tobacco smoking kill sperm cells.[9][10] Therefore, some governments require manufacturers to put warnings on packets. Smoking tobacco increases intake of cadmium, because the tobacco plant absorbs the metal. Cadmium, being chemically similar to zinc, may replace zinc in the DNA polymerase, which plays a critical role in sperm production. Zinc replaced by cadmium in DNA polymerase can be particularly damaging to the testes.[11]
DNA damage
Common inherited variants in genes that encode enzymes employed in DNA mismatch repair are associated with increased risk of sperm DNA damage and male infertility.[12] As men age there is a consistent decline in semen quality, and this decline appears to be due to DNA damage.[13] (Silva et al., 2012). These findings suggest that DNA damage is an important factor in male infertility.
Testicular factors
Testicular factors refer to conditions where the testes produce semen of low quantity and/or poor quality despite adequate hormonal support and include:
- Age
- Genetic defects on the Y chromosome
- Abnormal set of chromosomes
- Neoplasm, e.g. seminoma
- Idiopathic failure
- Cryptorchidism
- Varicocele (14% in one study)[14][15]
- Trauma
- Hydrocele
- Mumps[16]
- Malaria
- Testicular cancer
- Defects in USP26 in some cases[17]
- Acrosomal defects affecting egg penetration
- Idiopathic oligospermia - unexplained sperm deficiencies account for 30% of male infertility.[18]
Radiation therapy to a testis decreases its function, but infertility can efficiently be avoided by avoiding radiation to both testes.[19]
Post-testicular causes
Post-testicular factors decrease male fertility due to conditions that affect the male genital system after testicular sperm production and include defects of the genital tract as well as problems in ejaculation:
- Vas deferens obstruction
- Lack of Vas deferens, often related to genetic markers for Cystic Fibrosis
- Infection, e.g. prostatitis
- Retrograde ejaculation
- Ejaculatory duct obstruction
- Hypospadias
- Impotence
Diagnosis
The diagnosis of infertility begins with a medical history and physical exam by a physician or nurse practitioner. Typically two separate semen analyses will be required. The provider may order blood tests to look for hormone imbalances, medical conditions, or genetic issues.
Medical history
The history should include prior testicular or penile insults (torsion, cryptorchidism, trauma), infections (mumps orchitis, epididymitis), environmental factors, excessive heat,radiation, medications, and drug use (anabolic steroids, alcohol, smoking).
Sexual habits, frequency and timing of intercourse, use of lubricants, and each partner's previous fertility experiences are important.
The past medical or surgical history may reveal thyroid or liver disease (abnormalities of spermatogenesis), diabetic neuropathy (retrograde ejaculation), radical pelvic orretroperitoneal surgery (absent seminal emission secondary to sympathetic nerve injury), or hernia repair (damage to the vas deferens or testicular blood supply).
A family history may reveal genetic problems.
Physical examination
Usually, the patient disrobes completely and puts on a gown. The physician or NP will perform a thorough examination of the penis, scrotum, testicles, anus and rectum.
Sperm sample
The volume of the semen sample, approximate number of total sperm cells, sperm motility/forward progression, and % of sperm with normal morphology are measured. This is the most common type of fertility testing.[20][21] Semen deficiencies are often labeled as follows:
- Oligospermia or Oligozoospermia - decreased number of spermatozoa in semen
- Aspermia - complete lack of semen
- Hypospermia - reduced seminal volume
- Azoospermia - absence of sperm cells in semen
- Teratospermia - increase in sperm with abnormal morphology
- Asthenozoospermia - reduced sperm motility
There are various combinations of these as well, e.g. Teratoasthenozoospermia, which is reduced sperm morphology and motility. Low sperm counts are often associated with decreased sperm motility and increased abnormal morphology, thus the terms "oligoasthenoteratozoospermia" or "oligospermia" can be used as a catch-all.
Blood sample
Common hormonal test include determination of FSH and testosterone levels. A blood sample can reveal genetic causes of infertility, e.g. Klinefelter syndrome, a Y chromosome microdeletion, or cystic fibrosis.
Prevention
Some strategies suggested or proposed for avoiding male infertility include the following:
- Avoiding smoking[22] as it damages sperm DNA
- Avoiding heavy marijuana and alcohol use.[23]
- Avoiding excessive heat to the testes.[23]
- Sperm counts can be depressed by daily coital activity[23] and sperm motility may be depressed by coital activity that takes place too infrequently (abstinence 10–14 days or more).[23]
- When participating in contact sports, wear a Protective Cup and Jockstrap to protect the testicles. Sports such as Baseball, Football, Cricket, Lacrosse, Hockey, Softball,Paintball, Rodeo, Motorcross, Wrestling, Soccer, Karate or other Martial Arts or any sport where a ball, foot, arm, knee or bat can come into contact with the groin.
Treatment
Treatments vary according to the underlying disease and the degree of the impairment of the male fertility. Further, in an infertility situation, the fertility of the female needs to be considered.
Pre-testicular conditions can often be addressed by medical means or interventions.
Testicular-based male infertility tends to be resistant to medication. Usual approaches include using the sperm for intrauterine insemination (IUI), in vitro fertilization (IVF), or IVF with intracytoplasmatic sperm injection (ICSI). With IVF-ICSI even with a few sperm pregnancies can be achieved.
Obstructive causes of post-testicular infertility can be overcome with either surgery or IVF-ICSI. Ejaculatory factors may be treatable by medication, or by IUI therapy or IVF.
The off-label use of Clomiphene citrate, an anti-estrogen drug designed as a fertility medicine for women, is controversial.[24] Vitamin E helps counter oxidative stress,[25] which is associated with sperm DNA damage and reduced sperm motility.[26] A hormone-antioxidant combination may improve sperm count and motility.[27] The Low dose Estrogen Testosterone Combination Therapy may improve sperm count and motility in some men.[28] including severe oligospermia.[29][30]
Oral antioxidants e.g. brand name Maxoza-L given to males in couples undergoing in vitro fertilisation for male factor or unexplained subfertility result in significantly higher live birth rate.[31]
Female Infertility
Definition
Infertility means that couples have been trying to get pregnant with frequent intercourse for at least a year with no success. Female infertility, male infertility or a combination of the two affects millions of couples in the United States. An estimated 10 to 15 percent of couples have trouble getting pregnant or getting to a successful delivery.
Infertility results from female infertility factors about one-third of the time and male infertility factors about one-third of the time. In the rest, the cause is either unknown or a combination of male and female factors.
The cause of female infertility can be difficult to diagnose, but many treatments are available. Treatment options depend on the underlying problem. Treatment isn't always necessary — many infertile couples will go on to conceive a child spontaneously.
Symptoms
The main symptom of infertility is the inability of a couple to get pregnant. A menstrual cycle that's too long (35 days or more), too short (less than 21 days), irregular or absent can be a sign of lack of ovulation, which can be associated with female infertility. There may be no other outward signs or symptoms.
When to see a doctor
When to seek help depends, in part, on your age.
- If you're in your early 30s or younger, most doctors recommend trying to get pregnant for at least a year before having any testing or treatment.
- If you're between 35 and 40, discuss your concerns with your doctor after six months of trying.
- If you're older than 40, your doctor may want to begin testing or treatment right away.
Your doctor also may want to begin testing or treatment right away if you or your partner has known fertility problems, or if you have a history of irregular or painful periods, pelvic inflammatory disease, repeated miscarriages, prior cancer treatment, or endometriosis.
Causes
Multimedia
To become pregnant, each of these factors is essential:
- You need to ovulate.Achieving pregnancy requires that your ovaries produce and release an egg, a process known as ovulation. Your doctor can help evaluate your menstrual cycles and confirm ovulation.
- Your partner needs sperm. For most couples, this isn't a problem unless your partner has a history of illness or surgery. Your doctor can run some simple tests to evaluate the health of your partner's sperm.
- You need to have regular intercourse. You need to have regular sexual intercourse during your fertile time. Your doctor can help you better understand when you're most fertile during your cycle.
- You need to have open fallopian tubes and a normal uterus. The egg and sperm meet in the fallopian tubes, and the pregnancy needs a healthy place to grow.
For pregnancy to occur, every part of the complex human reproduction process has to take place just right. The steps in this process are as follows:
- One of the two ovaries releases a mature egg.
- The egg is picked up by the fallopian tube.
- Sperm swim up the cervix, through the uterus and into the fallopian tube to reach the egg for fertilization.
- The fertilized egg travels down the fallopian tube to the uterus.
- The fertilized egg implants and grows in the uterus.
In women, a number of factors can disrupt this process at any step. Female infertility is caused by one or more of these factors.
Ovulation disorders
Ovulation disorders, meaning you ovulate infrequently or not at all, account for infertility in about 25 percent of infertile couples. These can be caused by flaws in the regulation of reproductive hormones by the hypothalamus or the pituitary gland, or by problems in the ovary itself.
- Polycystic ovary syndrome (PCOS). In PCOS, complex changes occur in the hypothalamus, pituitary gland and ovaries, resulting in a hormone imbalance, which affects ovulation. PCOS is associated with insulin resistance and obesity, abnormal hair growth on the face or body, and acne. It's the most common cause of female infertility.
- Hypothalamic dysfunction. The two hormones responsible for stimulating ovulation each month — follicle-stimulating hormone (FSH) and luteinizing hormone (LH) — are produced by the pituitary gland in a specific pattern during the menstrual cycle. Excess physical or emotional stress, a very high or very low body weight, or a recent substantial weight gain or loss can disrupt this pattern and affect ovulation. The main sign of this problem is irregular or absent periods.
- Premature ovarian insufficiency. This disorder is usually caused by an autoimmune response where your body mistakenly attacks ovarian tissues or by premature loss of eggs from your ovary due to genetic problems or environmental insults such as chemotherapy. It results in the loss of the ability to produce eggs by the ovary, as well as a decreased estrogen production under the age of 40.
- Too much prolactin. Less commonly, the pituitary gland can cause excess production of prolactin (hyperprolactinemia), which reduces estrogen production and may cause infertility. Most commonly this is due to a problem in the pituitary gland, but it can also be related to medications you're taking for another disease.
Damage to fallopian tubes (tubal infertility)
When fallopian tubes become damaged or blocked, they keep sperm from getting to the egg or block the passage of the fertilized egg into the uterus. Causes of fallopian tube damage or blockage can include:
- Pelvic inflammatory disease, an infection of the uterus and fallopian tubes due to chlamydia, gonorrhea or other sexually transmitted infections
- Previous surgery in the abdomen or pelvis, including surgery for ectopic pregnancy, in which a fertilized egg becomes implanted and starts to develop in a fallopian tube instead of the uterus
- Pelvic tuberculosis, a major cause of tubal infertility worldwide, although uncommon in the United States
Endometriosis
Endometriosis occurs when tissue that normally grows in the uterus implants and grows in other locations. This extra tissue growth — and the surgical removal of it — can cause scarring, which may obstruct the tube and keep the egg and sperm from uniting. It can also affect the lining of the uterus, disrupting implantation of the fertilized egg. The condition also seems to affect fertility in less-direct ways, such as damage to the sperm or egg.
Uterine or cervical causes
Several uterine or cervical causes can impact fertility by interfering with implantation or increasing the likelihood of a miscarriage.
- Benign polyps or tumors (fibroids or myomas) are common in the uterus, and some types can impair fertility by blocking the fallopian tubes or by disrupting implantation. However, many women who have fibroids or polyps can become pregnant.
- Endometriosis scarring or inflammation within the uterus can disrupt implantation.
- Uterine abnormalities present from birth, such as an abnormally shaped uterus, can cause problems becoming or remaining pregnant.
- Cervical stenosis, a cervical narrowing, can be caused by an inherited malformation or damage to the cervix.
- Sometimes the cervix can't produce the best type of mucus to allow the sperm to travel through the cervix into the uterus.
Risk factors
Certain factors may put you at higher risk of infertility, including:
- Age. With increasing age, the quality and quantity of a woman's eggs begin to decline. In the mid-30s, the rate of follicle loss accelerates, resulting in fewer and poorer quality eggs, making conception more challenging and increasing the risk of miscarriage.
- Smoking. Besides damaging your cervix and fallopian tubes, smoking increases your risk of miscarriage and ectopic pregnancy. It's also thought to age your ovaries and deplete your eggs prematurely, reducing your ability to get pregnant. Stop smoking before beginning fertility treatment.
- Weight. If you're overweight or significantly underweight, it may hinder normal ovulation. Getting to a healthy body mass index (BMI) has been shown to increase the frequency of ovulation and likelihood of pregnancy.
- Sexual history. Sexually transmitted infections such as chlamydia and gonorrhea can cause fallopian tube damage. Having unprotected intercourse with multiple partners increases your chances of contracting a sexually transmitted disease (STD) that may cause fertility problems later.
- Alcohol. Heavy drinking is associated with an increased risk of ovulation disorders and endometriosis.
Tests and diagnosis
If you've been unable to conceive within a reasonable period of time, seek help from your doctor for further evaluation and treatment of infertility.
Fertility tests may include:
- Ovulation testing. An over-the-counter ovulation prediction kit — a test that you can perform at home — detects the surge in luteinizing hormone (LH) that occurs before ovulation. If you have not had positive home ovulation tests, a blood test for progesterone — a hormone produced after ovulation — can document that you're ovulating. Other hormone levels, such as prolactin, also may be checked.
- Hysterosalpingography. During hysterosalpingography (his-tur-o-sal-ping-GOG-ruh-fee), X-ray contrast is injected into your uterus and an X-ray is taken to determine if the uterine cavity is normal and whether the fluid passes out of the uterus and spills out of your fallopian tubes. If abnormalities are found, you'll likely need further evaluation. In a few women, the test itself can improve fertility, possibly by flushing out and opening the fallopian tubes.
- Ovarian reserve testing. This testing helps determine the quality and quantity of eggs available for ovulation. Women at risk of a depleted egg supply — including women older than 35 — may have this series of blood and imaging tests.
- Other hormone testing. Other hormone tests check levels of ovulatory hormones as well as thyroid and pituitary hormones that control reproductive processes.
- Imaging tests. Pelvic ultrasound looks for uterine or fallopian tube disease. Sometimes a hysterosonography (his-tur-o-suh-NOG-ruh-fee) is used to see details inside the uterus that are not seen on a regular ultrasound.
Depending on your situation, rarely your testing may include:
- Other imaging tests. Depending on your symptoms, your doctor may request a hysteroscopy to look for uterine or fallopian tube disease.
- Laparoscopy. This minimally invasive surgery involves making a small incision beneath your navel and inserting a thin viewing device to examine your fallopian tubes, ovaries and uterus. Laparoscopy may identify endometriosis, scarring, blockages or irregularities of the fallopian tubes, and problems with the ovaries and uterus.
- Genetic testing. Genetic testing helps determine whether there's a genetic defect causing infertility.
Prevention
If you're a woman thinking about getting pregnant soon or in the future, you may improve your chances of having normal fertility if you:
- Maintain a normal weight. Overweight and underweight women are at increased risk of ovulation disorders. If you need to lose weight, exercise moderately. Strenuous, intense exercise of more than seven hours a week has been associated with decreased ovulation.
- Quit smoking. Tobacco has multiple negative effects on fertility, not to mention your general health and the health of a fetus. If you smoke and are considering pregnancy, quit now.
- Avoid alcohol. Heavy alcohol use may lead to decreased fertility. And any alcohol use can affect the health of a developing fetus. If you're planning to become pregnant, avoid alcohol, and don't drink alcohol while you're pregnant.
- Reduce stress. Some studies have shown that couples experiencing psychological stress had poorer results with infertility treatment. If you can, find a way to reduce stress in your life before trying to become pregnant.
- Limit caffeine. Some physicians suggest limiting caffeine intake to less than 200 to 300 milligrams a day.
Azoospermia
Not to be confused with aspermia, which refers to the absence of semen in a male.
| Azoospermia | |
|---|---|
| Classification and external resources | |

Semen analysis revealing no sperm cells and multiple white blood cells
| |
Azoospermia is the medical condition of a man not having any measurable level of sperm in his semen. It is associated with very low levels of fertility or even sterility, but many forms are amenable to medical treatment. In humans, azoospermia affects about 1% of the male population[1] and may be seen in up to 20% of male infertility situations.[2]
Classification
Azoospermia can be classified into three major types as listed.[2] Many conditions listed may also cause various degrees ofoligospermia rather than azoospermia.
Pretesticular azoospermia
Pretesticular azospermia is characterized by inadequate stimulation of otherwise normal testicles and genital tract. Typically, follicle-stimulating hormone (FSH) levels are low (hypogonadotropic) commensurate with inadequate stimulation of the testes to produce sperm. Examples include hypopituitarism (for various causes), hyperprolactinemia, and exogenous FSH suppression by testosterone.Chemotherapy may suppress spermatogenesis.[3] Pretesticular azoospermia is seen in about 2% of azoospermia[2]
Testicular azoospermia
In this situation the testes are abnormal, atrophic, or absent, and sperm production severely disturbed to absent. FSH levels tend to be elevated (hypergonadotropic) as the feedback loop is interrupted. The condition is seen in 49-93% of men with azoospermia.[2] Testicular failure includes absence of failure production as well as low production and maturation arrest during the process of spermatogenesis.
Causes for testicular failure include congenital issues such as in certain genetic conditions (e.g. Klinefelter syndrome), some cases of cryptorchidism or Sertoli cell-only syndromeas well as acquired conditions by infection (orchitis), surgery (trauma, cancer), radiation,[3] or other causes. Mast cells releasing inflammatory mediators appear to directly suppress sperm motility in a potentially reversible manner, and may be a common pathophysiological mechanism for many causes leading to inflammation.[4]
Generally, men with unexplained hypergonadotropic azoospermia need to undergo a chromosomal evaluation.
Posttesticular azoospermia
In posttesticular azoospermia sperm are produced but not ejaculated, a condition that affects 7-51% of azoospermic men.[2] The main cause is a physical obstruction (obstructive azoospermia) of the posttesticular genital tracts. The most common reason is a vasectomy done to induce contraceptive sterility.[5] Other obstructions can be congenital (example agenesis of the vas deferens as seen in certain cases of cystic fibrosis) or acquired, such as ejaculatory duct obstruction for instance by infection.
Ejaculatory disorders include retrograde ejaculation and anejaculation; in these conditions sperm are produced but not expelled.
Idiopathic azoospermia
Idiopathic azoospermia is where there is no known cause of the condition. It may be a result of multiple risk factors, such as age and weight. For example, a review in 2013 came to the result that oligospermia and azoospermia are significantly associated with being overweight (odds ratio 1.1), obese (odds ratio 1.3) and morbidly obese (odds ratio 2.0), but the cause of this is unknown.[6] The review found no significant relation between oligospermia and being underweight.[6]
Diagnosis and evaluation
Azoospermia is usually detected in the course of an infertility investigation. It is established on the basis of two semen analysis evaluations done at separate occasions (when the seminal specimen after centrifugation shows no sperm under the microscope) and requires a further work-up. The following work-up is recommended by the Canadian Urologic Association:[2]
The investigation includes a history, a physical examination including a thorough evaluation of the scrotum and testes, laboratory tests, and possibly imaging. History includes the general health, sexual health, past fertility, libido, and sexual activity. Past exposure to a number of agents needs to be queried including medical agents like hormone/steroid therapy, antibiotics (sulphasalazine), alpha-blockers, 5 alpha-reductase inhibitors, chemotherapeutic agents, pesticides, recreational drugs (marijuana, excessive alcohol), and heat exposure of the testes. A history of surgical procedures of the genital system needs to be elicited. The family history needs to be assessed to look for genetic abnormalities.
Absence of the vas deferens may be detectable on physical examination and can be confirmed by a transrectal ultrasound (TRUS). If confirmed genetic testing for cystic fibrosis is in order. Retrograde ejaculation is diagnosed by examining a postejaculatory urine for presence of sperm after making it alkaline and centifuging it.[7]
Low levels of LH and FSH with low or normal testosterone levels are indicative of pretesticular problems, while high levels of gonadotropins indicate testicular problems. However, often this distinction is not clear and the differentiation between obstructive versus non-obstructive azoospermia may require a testicular biopsy.
Serum inhibin-B weakly indicates presence of sperm cells in the testes, raising chances for successfully achieving pregnancy through testicular sperm extraction (TESE), although the association is not very substantial, having a sensitivity of 0.65 (95% confidence interval [CI]: 0.56–0.74) and a specificity of 0.83 (CI: 0.64–0.93) for prediction the presence of sperm in the testes in non-obstructive azoospermia.[8]
Seminal plasma proteins TEX101 and ECM1 were recently proposed for the differential diagnosis of azoospermia forms and subtypes, and for prediction of TESE outcome.[9]
It is recommended that men primary hypopituitarism may be linked to a genetic cause, a genetic evaluation is indicated in men with azoospermia due to primary hypopituitarism.[1]Azoospermic men with testicular failure are advised to undergo karyotype and Y-micro-deletion testing.[10][11]
Genetic causes of azoospermia
Genetic factors can cause pretesticular, testicular, and posttesticular azoospermia (or oligospermia) and include the following situations:[11] The frequency of chromosomal abnormalities is inversely proportional to the semen count, thus males with azoospermia are at risk to have a 10-15% (other sources citing 15-20% incidence[7]) abnormalities on karyotyping versus about <1 % in the fertile male population.[1]
Pretesticular azoospermia may be caused by congential hypopituitarism, Kallmann syndrome, Prader-Willi syndrome and other genetic conditions that lead to GnRH orgonadotropin deficiency. Testicular azoospermia is seen in Klinefelter syndrome(XXY) and the XX male syndrome. In addition, 13% of men with azoospermia have a defective spermatogenesis that is linked to defects of the Y chromosome.[11] Such defects tend to be de novo micro-deletions and affect usually the long arm of the chromosome. A section of the long arm of the Y chromosome has been termed Azoospermia Factor (AZF) at Yq11 and subdivided into AZFa, AZFb, AZFc and possibly more subsections. Defects in this area can lead to oligospermia or azoospermia, however, a tight genotype-phenotype correlation has not been achieved.[11] Spermatogenesis is defective with gene defects for theandrogen receptor.
Posttesticular azoospermia can be seen with certain point mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene commonly associated with congenital vas deferens abnormalities.
Genetic counselling is indicated for men with genetic causes of azoospermia. In terms of reproduction, it needs to be considered if the genetic defect could be transmitted to the offspring.
Oligospermia
| Oligozoospermia | |
|---|---|
| Classification and external resources | |
Oligozoospermia, refers to semen with a low concentration of sperm[1] and is a common finding in male infertility. Often semen with a decreased sperm concentration may also show significant abnormalities in sperm morphology and motility (technically "oligoasthenoteratozoospermia"). There has been interest in replacing the descriptive terms used in semen analysis with more quantitative information.[2]
Diagnosis[
The diagnosis of oligozoospermia is based on one low count in a semen analysis performed on two occasions. For many decades sperm concentrations of less than 20 million sperm/ml were considered low or oligospermic, recently, however, the WHO reassessed sperm criteria and established a lower reference point, less than 15 million sperm/ml, consistent with the 5th percentile for fertile men.[3] Sperm concentrations fluctuate and oligospermia may be temporary or permanent.
Sources usually classify oligospermia in 3 classes:[4]
- Mild: concentrations 10 million - 20 million sperm/ml
- Moderate: concentrations 5 million - 10 million sperm/ml
- Severe: concentrations less than 5 million sperm/ml
The diagnosis of oligozoospermia requires a work-up via semen analysis (listed in Male infertility).
Causes
There are many causes for oligospermia including:
Pre-testicular causes
Pre-testicular factors refer to conditions that impede adequate support of the testes and include situations of poor hormonal support and poor general health including:
- Hypogonadism due to various causes
- Drugs, alcohol, smoking
- Strenuous riding (bicycle riding,[6] horseback riding)
- Medications, including androgens.
Testicular factors
Testicular factors refer to conditions where the testes produces semen of poor quality despite adequate hormonal support and include:
- Age
- Genetic defects on the Y chromosome
- Abnormal set of chromosomes
- Neoplasm, e.g. seminoma
- Cryptorchidism
- Varicocele (14% in one study)[7][8]
- Trauma
- Hydrocele
- Mumps[9]
- Malaria
- Defects in USP26 in some cases[10]
Mast cells releasing inflammatory mediators appear to directly suppress sperm motility in a potentially reversible manner, and may be a common pathophysiological mechanism for several of the above mentioned factors.[11]
Post-testicular causes
Post-testicular factors decrease male fertility due to conditions that affect the male genital system after testicular sperm production and include defects of the genital tract as well as problems in ejaculation:
- Vas deferens obstruction
- Lack of Vas deferens, often related to genetic markers for Cystic Fibrosis
- Infection, e.g. prostatitis
- Ejaculatory duct obstruction
Idiopathic oligospermia (oligoasthenoteratozoospermia)
In about 30% of infertile men no causative factor is found for their decrease in sperm concentration or quality by common clinical, instrumental, or laboratory means, and the condition is termed "idiopathic" (unexplained).[12] A number of factors may be involved in the genesis of this condition, including age, infectious agents ( such as Chlamydia trachomatis), Y chromosome microdeletions, mitochondrial changes, environmental pollutants, and "subtle" hormonal changes.[12]
A review in 2013 came to the result that oligospermia and azoospermia are significantly associated with being overweight (odds ratio 1.1), obese (odds ratio 1.3) and morbidly obese (odds ratio 2.0), but the cause of this is unknown.[13] It found no significant relation between oligospermia and being underweight.[13]
Fertility
Achieving a pregnancy naturally may be a challenge if the male suffers from a low sperm count. However, chances are good if the female partner is fertile; many couples with this problem have been successful. Prognosis is more limited if there is a combination of factors that include sperm dysfunction and reduced ovarian reserve.
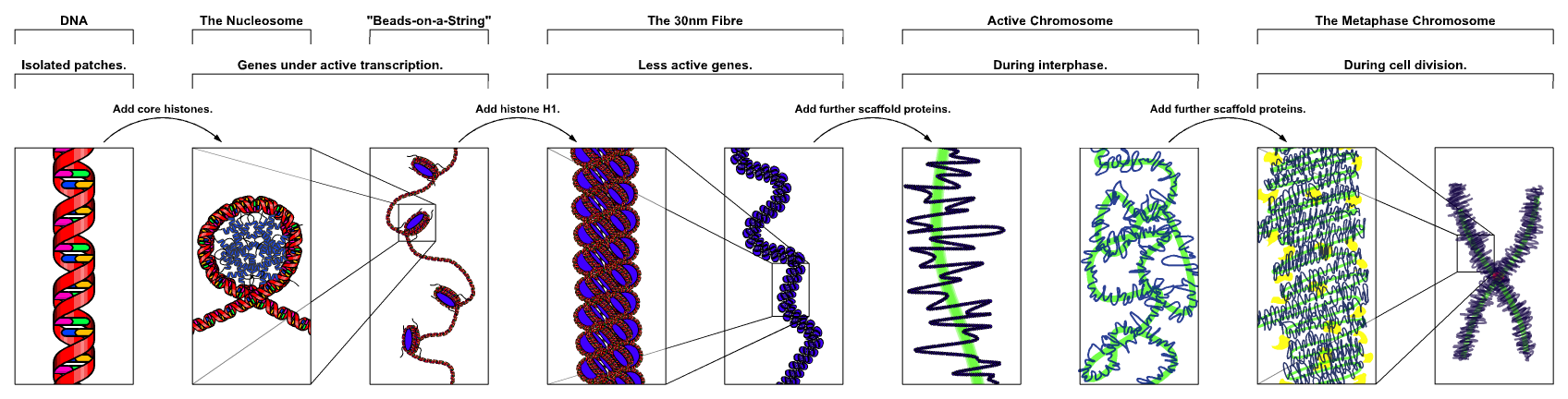
Spermatogenesis
| Spermatogenesis | |
|---|---|

Seminiferous tubule with maturing sperm. H&E stain.
| |

A mature human Spermatozoon
|
Spermatogenesis is the process in which spermatozoa are produced from male primordial germ cells by way of mitosis and meiosis. The initial cells in this pathway are called spermatogonia, which yield primary spermatocytes by mitosis. The primary spermatocyte divides meiotically into two secondary spermatocytes; each secondary spermatocyte then completes meiosis as it divides into twospermatids. These develop into mature spermatozoa, also known as sperm cells. Thus, the primary spermatocyte gives rise to two cells, the secondary spermatocytes, and the two secondary spermatocytes by their subdivision produce four spermatozoa.[1]
Spermatozoa are the mature male gametes in many sexually reproducing organisms. Thus, spermatogenesis is the male version ofgametogenesis. In mammals it occurs in the male testes and epididymis in a stepwise fashion. Spermatogenesis is highly dependent upon optimal conditions for the process to occur correctly, and is essential for sexual reproduction. DNA methylation and histone modification have been implicated in the regulation of this process.[2] It starts at puberty and usually continues uninterrupted until death, although a slight decrease can be discerned in the quantity of produced sperm with increase in age (see Male infertility).
Purpose
Spermatogenesis produces mature male gametes, commonly called sperm but specifically known as spermatozoa, which are able to fertilize the counterpart female gamete, the oocyte, during conception to produce a single-celled individual known as a zygote. This is the cornerstone of sexual reproduction and involves the two gametes both contributing half the normal set of chromosomes (haploid) to result in a chromosomally normal (diploid) zygote.
To preserve the number of chromosomes in the offspring – which differs between species – each gamete must have half the usual number of chromosomes present in other body cells. Otherwise, the offspring will have twice the normal number of chromosomes, and serious abnormalities may result. In humans, chromosomal abnormalities arising from incorrect spermatogenesis can result in Down Syndrome, Klinefelter's Syndrome, andspontaneous abortion.
Location
Spermatogenesis takes place within several structures of the male reproductive system. The initial stages occur within the testes and progress to the epididymis where the developing gametes mature and are stored until ejaculation. The seminiferous tubules of the testes are the starting point for the process, where stem cells adjacent to the inner tubule wall divide in a centripetal direction—beginning at the walls and proceeding into the innermost part, or lumen—to produce immature sperm. Maturation occurs in the epididymis.
Duration
For humans, entire process of spermatogenesis takes 74 days. Including the transport on ductal system, it takes 3 months. Testes produce 200 to 300 million spermatozoa daily.[3]
Stages
The entire process of spermatogenesis can be broken up into several distinct stages, each corresponding to a particular type of cell in human. In the following table, ploidy, copy number and chromosome/chromatid counts are for one cell, generally prior to DNA synthesis and division (in G1 if applicable). The primary spermatocyte is arrested after DNA synthesis and prior to division.
| Cell type | ploidy/chromosomes in human | DNA copy number/chromatids in human | Process entered by cell |
| spermatogonium (types Ad, Ap and B) | diploid (2N) / 46 | 2C / 46 | spermatocytogenesis (mitosis) |
| primary spermatocyte | diploid (2N) / 46 | 4C / 2x46 | spermatidogenesis (meiosis 1) |
| two secondary spermatocytes | haploid (N) / 23 | 2C / 46 | spermatidogenesis (meiosis 2) |
| four spermatids | haploid (N) / 23 | 1C / 23 | spermiogenesis |
| four functional spermatozoids | haploid (N) / 23 | 1C / 23 | spermiation |
Spermatocytogenesis
| It has been suggested that this article be merged with Spermatocytogenesis. (Discuss) Proposed since January 2012. |
Main article: Spermatocytogenesis
Spermatocytogenesis is the male form of gametocytogenesis and results in the formation ofspermatocytes possessing half the normal complement of genetic material. In spermatocytogenesis, a diploid spermatogonium, which resides in the basal compartment of the seminiferous tubules, divides mitotically, producing two diploid intermediate cells calledprimary spermatocytes. Each primary spermatocyte then moves into the adluminal compartment of the seminiferous tubules and duplicates its DNA and subsequently undergoesmeiosis I to produce two haploid secondary spermatocytes, which will later divide once more into haploid spermatids. This division implicates sources of genetic variation, such as random inclusion of either parental chromosomes, and chromosomal crossover, to increase the genetic variability of the gamete.
Each cell division from a spermatogonium to a spermatid is incomplete; the cells remain connected to one another by bridges of cytoplasm to allow synchronous development. It should also be noted that not all spermatogonia divide to produce spermatocytes; otherwise, the supply of spermatogonia would run out. Instead, certain types of spermatogonia divide mitotically to produce copies of themselves, ensuring a constant supply of spermatogonia to fuel spermatogenesis.[4]
Spermatidogenesis
Main article: Spermatidogenesis
Spermatidogenesis is the creation of spermatids from secondary spermatocytes. Secondary spermatocytes produced earlier rapidly enter meiosis II and divide to produce haploid spermatids. The brevity of this stage means that secondary spermatocytes are rarely seen in histological studies.
Spermiogenesis
Main article: Spermiogenesis
During spermiogenesis, the spermatids begin to grow a tail, and develop a thickened mid-piece, where the microtubules gather and form an axoneme. Spermatid DNA also undergoes packaging, becoming highly condensed. The DNA is packaged firstly with specific nuclear basic proteins, which are subsequently replaced with protamines during spermatid elongation. The resultant tightly packedchromatin is transcriptionally inactive. The Golgi apparatus surrounds the now condensed nucleus, becoming the acrosome. One of the centrioles of the cell elongates to become the tail of the sperm.
Maturation then takes place under the influence of testosterone, which removes the remaining unnecessary cytoplasm andorganelles. The excess cytoplasm, known as residual bodies, is phagocytosed by surrounding Sertoli cells in the testes. The resulting spermatozoa are now mature but lack motility, rendering them sterile. The mature spermatozoa are released from the protectiveSertoli cells into the lumen of the seminiferous tubule in a process called spermiation.
The non-motile spermatozoa are transported to the epididymis in testicular fluid secreted by the Sertoli cells with the aid of peristaltic contraction. While in the epididymis the spermatozoa gain motility and become capable of fertilization. However, transport of the mature spermatozoa through the remainder of the male reproductive system is achieved via muscle contraction rather than the spermatozoon's recently acquired motility.
Role of Sertoli cells
Main article: Sertoli cell
At all stages of differentiation, the spermatogenic cells are in close contact with Sertoli cells which are thought to provide structural and metabolic support to the developing sperm cells. A single Sertoli cell extends from the basement membrane to the lumen of the seminiferous tubule, although the cytoplasmic processes are difficult to distinguish at the light microscopic level.
Sertoli cells serve a number of functions during spermatogenesis, they support the developing gametes in the following ways:
- Maintain the environment necessary for development and maturation, via the blood-testis barrier
- Secrete substances initiating meiosis
- Secrete supporting testicular fluid
- Secrete androgen-binding protein (ABP), which concentrates testosterone in close proximity to the developing gametes
- Testosterone is needed in very high quantities for maintenance of the reproductive tract, and ABP allows a much higher level of fertility
- Secrete hormones affecting pituitary gland control of spermatogenesis, particularly the polypeptide hormone, inhibin
- Phagocytose residual cytoplasm left over from spermiogenesis
- They release Antimullerian hormone which prevents formation of the Müllerian Duct / Oviduct.
- Protect spermatids from the immune system of the male, via the blood-testis barrier
The intercellular adhesion molecules ICAM-1 and soluble ICAM-1 have antagonistic effects on the tight junctions forming the blood-testis barrier.[5] ICAM-2 molecules regulate spermatid adhesion on the apical side of the barrier (towards the lumen).[5] during spermatogenesis, the cells are closely associated with sertoli cells which lies at regular interval alone the seminiferous tubules. sertoli cells perform the following tasks:
- are target cells for follicular stimulating hormones (FSH)
- synthesize an androgen binding protein that maintain a high levels of testosterone inside the seminiferous tubules
- maintain the blood-testes barrier which protect the body's immune system from destroying the developing sperm cells
- create an environment that is necessary in the differentiation of sperm cell
- degrade the residual cytoplasm that is shed during spermatogenesis.
Influencing factors
The process of spermatogenesis is highly sensitive to fluctuations in the environment, particularly hormones and temperature. Testosterone is required in large local concentrations to maintain the process, which is achieved via the binding of testosterone by androgen binding protein present in the seminiferous tubules. Testosterone is produced by interstitial cells, also known as Leydig cells, which reside adjacent to the seminiferous tubules.
Seminiferous epithelium is sensitive to elevated temperature in humans and some other species, and will be adversely affected by temperatures as high as normal body temperature. Consequently, the testes are located outside the body in a sack of skin called the scrotum. The optimal temperature is maintained at 2 °C (man)–8 °C (mouse) below body temperature. This is achieved by regulation of blood flow[6] and positioning towards and away from the heat of the body by the cremasteric muscle and the dartos smooth muscle in the scrotum.
Dietary deficiencies (such as vitamins B, E and A), anabolic steroids, metals (cadmium and lead), x-ray exposure, dioxin, alcohol, and infectious diseases will also adversely affect the rate of spermatogenesis.[citation needed] In addition, the male germ line is susceptible to DNA damage caused by oxidative stress, and this damage likely has a significant impact on fertilization and pregnancy.[7] Exposure to pesticides also affects spermatogenesis. [8]
Hormonal control
Hormonal control of spermatogenesis varies among species. In humans the mechanism is not completely understood, however it is known that initiation of spermatogenesis occurs at puberty due to the interaction of the hypothalamus, pituitary gland and Leydig cells. If the pituitary gland is removed, spermatogenesis can still be initiated by follicle stimulating hormone and testosterone.
Follicle stimulating hormone stimulates both the production of androgen binding protein by Sertoli cells, and the formation of the blood-testis barrier. Androgen binding protein is essential to concentrating testosterone in levels high enough to initiate and maintain spermatogenesis, which can be 20–50 times higher than the concentration found in blood. Follicle stimulating hormone may initiate the sequestering of testosterone in the testes, but once developed only testosterone is required to maintain spermatogenesis. However, increasing the levels of follicle stimulating hormone will increase the production of spermatozoa by preventing the apoptosis of type A spermatogonia. The hormone inhibin acts to decrease the levels of follicle stimulating hormone. Studies from rodent models suggest that gonadotropin hormones (both LH and FSH) support the process of spermatogenesis by suppressing the proapoptotic signals and therefore promote spermatogenic cell survival.[9]
The Sertoli cells themselves mediate parts of spermatogenesis through hormone production. They are capable of producing the hormones estradiol and inhibin. The Leydig cells are also capable of producing estradiol in addition to their main product testosterone.
Fallopian tube
| Fallopian Tubes | |
|---|---|

Schematic frontal view of female anatomy
| |

Vessels of the uterus and its appendages, rear view. (Fallopian tubes visible at top right and top left.)
| |
| Latin | Tuba uterina (Greek: Salpinx) |
| Artery | tubal branches of ovarian artery,tubal branch of uterine artery viamesosalpinx |
| Lymph | lumbar lymph nodes |
| Precursor | Müllerian duct |
The Fallopian tubes, also known as oviducts, uterine tubes, and salpinges (singular salpinx) are two very fine tubes lined withciliated epithelia, leading from the ovaries of female mammals into the uterus, via the utero-tubal junction. In non-mammalian vertebrates, the equivalent structures are the oviducts.
History
They are named after their discoverer, the 16th century Italian anatomist, Gabriele Falloppio.
Though the name 'Fallopian tube' is eponymous, some texts spell it with a lower case 'f' from the assumption that the adjective 'fallopian' has been absorbed into modern English as the de facto name for the structure.
Structure
In a woman's body the tube allows passage of the egg from the ovary to the uterus. Its different segments are (lateral to medial): theinfundibulum with its associated fimbriae near the ovary, the ampullary region that represents the major portion of the lateral tube, theisthmus which is the narrower part of the tube that links to the uterus, and the interstitial (also known as intramural) part that transverses the uterine musculature. The tubal ostium is the point where the tubal canal meets the peritoneal cavity, while the uterine opening of the Fallopian tube is the entrance into the uterine cavity, the utero-tubal junction.
Histology
A cross section of Fallopian tube shows four distinct layers: Serosa, subserosa, lamina propria and innermost mucosal layer. The serosa is derived from visceral peritoneum. Subserosa is composed of loose adventitious tissue, blood vessels, lymphatics, an outer longitudinal and inner circular smooth muscle coats. This layer is responsible for peristaltic action of fallopian tube. Lamina propria is a vascular connective tissue.[1] There are two types of cells within the simple columnar epithelium of the Fallopian tube (oviduct). Ciliated cells predominate throughout the tube, but are most numerous in the infundibulum and ampulla. Estrogen increases the production of cilia on these cells. Interspersed between the ciliated cells are peg cells, which contain apical granules and produce the tubular fluid. This fluid contains nutrients for spermatozoa, oocytes, and zygotes. The secretions also promote capacitation of the sperm by removing glycoproteins and other molecules from the plasma membrane of the sperm. Progesterone increases the number of peg cells, while estrogen increases their height and secretory activity. Tubal fluid flows against the action of the ciliae, that is toward the fimbrial end.
In view of longitudinal variation in histological features of tube, the isthmus has thick muscular coat and simple mucosal folds; whereas ampulla has complex mucosal folds.[1][clarification needed]
Development
Embryos have two pairs of ducts to let gametes out of the body; one pair (the Müllerian ducts) develops in females into the Fallopian tubes, uterus and vagina, while the other pair (the Wolffian ducts) develops in males into the epididymis and vas deferens.
Normally, only one of the pairs of tubes will develop while the other regresses and disappears in utero.
The homologous organ in the male is the rudimentary appendix testis.
Function
Fertilization
When an oocyte is developing in an ovary, it is encapsulated in a spherical collection of cells known as an ovarian follicle. Just prior to ovulation the primary oocyte completes meiosis I to form the first polar body and a secondary oocyte which is arrested in metaphase of meiosis II. This secondary oocyte is then ovulated. The follicle and the ovary's wall rupture, allowing the secondary oocyte to escape. The secondary oocyte is caught by the fimbriated end and travels to the ampulla of the uterine tube where typically the sperm are met and fertilization occurs; meiosis II is promptly completed. The fertilized ovum, now a zygote, travels towards the uterus aided by activity of tubal cilia and activity of the tubal muscle. After about five days the new embryo enters the uterine cavity and on about the sixth day implants on the wall of the uterus.
The release of an oocyte does not alternate between the two ovaries and seems to be random. After removal of an ovary, the remaining one produces an egg every month.[2]
Occasionally the embryo implants into the Fallopian tube instead of the uterus, creating an ectopic pregnancy, commonly known as a "tubal pregnancy".
Clinical significance
Patency testing
While a full testing of tubal functions in patients with infertility is not possible, testing of tubal patency is important as tubal obstruction is a major cause of infertility. Ahysterosalpingogram, laparoscopy and dye, or HyCoSy will demonstrate that tubes are open. Tubal insufflation is a standard procedure for testing patency. During surgery the condition of the tubes may be inspected and a dye such as methylene blue can be injected into the uterus and shown to pass through the tubes when the cervix is occluded. As tubal disease is often related to Chlamydia infection, testing for Chlamydia antibodies has become a cost-effective screening device for tubal pathology.[3]
Inflammation
Salpingitis is inflammation of the Fallopian tubes and may be found alone, or be a component of pelvic inflammatory disease(PID). Saccular dilation of the fallopian tube at its narrow portion, due to inflammation, is known as salpingitis isthmica nodosa. Like PID and endometriosis, it may lead to Fallopian tube obstruction. Fallopian tube obstruction is associated with infertilityand ectopic pregnancy.
Cancer
Fallopian tube cancer, which typically arises from the epithelial lining of the Fallopian tube, has historically been considered to be a very rare malignancy. Recent evidence suggests it probably represents a significant portion of what has been classified as ovarian cancer in the past.[4] While tubal cancers may be misdiagnosed as ovarian cancer, it is of little consequence as the treatment of both ovarian and Fallopian tube cancer is similar.
Surgery
The surgical removal of a Fallopian tube is called a salpingectomy. To remove both sides is a bilateral salpingectomy. An operation that combines the removal of a Fallopian tube with removal of at least one ovary is a salpingo-oophorectomy. An operation to remove a fallopian tube obstruction is called a tuboplasty.
Fallopian tube obstruction (Blockage)
| Fallopian tube obstruction | |
|---|---|
| Classification and external resources | |

The presence of a hydrosalpinx by sonography indicates distal tubal obstruction
| |
| ICD-10 | N97.1 |
| ICD-9 | 628.2 |
Fallopian tube obstruction is a major cause of female infertility. Blocked fallopian tubes are unable to let the ovum and the spermconverge, thus making fertilization impossible. Fallopian Tubes are also known as oviducts, uterine tubes, and salpinges (singular salpinx).
Types
Approximately 20% of female infertility can be attributed to tubal causes. Distal tubal occlusion (affecting the end towards the ovary) is typically associated with hydrosalpinx formation and often caused by Chlamydia trachomatis.[1] Pelvic adhesions may be associated with such an infection. In less severe forms, the fimbriae may be aggluntinated and damaged, but some patency may still be preserved. Midsegment tubal obstruction can be due to tubal ligation procedures as that part of the tube is a common target of sterilization interventions. Proximal tubal occlusion can occur after infection such as a septic abortion. Also, some tubal sterilization procedures such as the Essure procedure target the part of the tube that is near the uterus..
Causes[edit]
Most commonly a tube may be obstructed due to infection such as pelvic inflammatory disease (PID). The rate of tubal infertility has been reported to be 12% after one, 23% after two, and 53% after three episodes of PID.[1] The Fallopian tubes may also be occluded or disabled by endometritis, infections after childbirth and intraabdominal infections including appendicitis and peritonitis. The formation of adhesions may not necessarily block a fallopian tube, but render it dysfunctional by distorting or separating it from the ovary. It has been reported that women with distal tubal occlusion have a higher rate of HIV infection.[2]
Fallopian tubes may be blocked as a method of contraception. In these situations tubes tend to be healthy and typically patients requesting the procedure had children. Tubal ligation is considered a permanent procedure.
Evaluation
While a full testing of tubal functions in patients with infertility is not possible, testing of tubal patency is feasible. A hysterosalpingogram will demonstrate that tubes are open when the radioopaque dye spills into the abdominal cavity. Sonography can demonstrate tubal abnormalities such as a hydrosalpinx indicative of tubal occlusion. During surgery, typically laparoscopy, the status of the tubes can be inspected and a dye such as methylene blue can be injected in a process termed chromotubation into the uterus and shown to pass through the tubes when the cervix is occluded. Laparoscopic chromotubation has been described as the gold standard of tubal evaluation.[3] As tubal disease is often related to Chlamydia infection, testing for Chlamydia antibodies has become a cost-effective screening device for tubal pathology.[3]
Tubal insufflation is only of historical interest as an older office method to indicate patency;[4] it was used prior to laparoscopic evaluation of pelvic organs.
Treatment
Treatment of fallopian tube obstruction has traditionally been treated with fallopian tubal surgery (tuboplasty) with a goal of restoring patency to the tubes and thus possibly normal function. A common modern day method of treatment is in vitro fertilization as it is more cost-effective, less invasive, and results are immediate. Alternative methods such asmanual physical therapy are also cited for the ability to open and return function to blocked fallopian tubes in some women. Treatments such as assisted reproductive technologies are used more often than surgery.[5]
Tuboplasty[edit]
Main article: Tuboplasty
Tuboplasty refers to a number of surgical operations that attempt to restore patency and functioning of the Fallopian tube(s) so that a pregnancy could be achieved. As tubal infertility is a common cause of infertility, tuboplasties were commonly performed prior to the development of effective in vitro fertilization (IVF).
Different types of tuboplasty have been developed and can be applied by laparoscopy or laparotomy.[6] They include lysis of adhesions,[7] fimbrioplasty (repairing the fimbriated end of the tubes),[8] salpinostomy (creating an opening for the tube), resection and reananstomosis (removing a piece of blocked tube and reuniting the remaining patent parts of the tube), and tubal reimplantation (reconnecting the tube to the uterus). Further, using fluoroscopy or hysteroscopy proximal tubal occlusion can be overcome by unilateral or bilateral selective tubal cannulation, a procedure where a thin catheter is advanced through the proximal portion of the fallopian tube os to examine and possibly restore tubal patency[8] salpinostomy (creating an opening for the tube)[9] or falloposcopy.
Results of tubal surgery are inversely related to damage that exists prior to surgery.[10] Development of adhesions remains a problem.[1] Patients with operated tubes are at increased risk for ectopic pregnancy.,[10] although in vitro fertiliztion in patients with damaged tubes is also associated with a risk for ectopic pregnancy.
In vitro fertilization
In vitro fertilisation is a process by which an egg is fertilised by sperm outside the body: in vitro. IVF is a major treatment for infertility when other methods of assisted reproductive technology have failed. The process involves monitoring a woman's ovulatory process, removing ovum or ova (egg or eggs) from the woman's ovaries and letting sperm fertilise them in a fluid medium in a laboratory. When a woman's natural cycle is monitored to collect a naturally selected ovum (egg) for fertilisation, it is known as natural cycle IVF. The fertilised egg (zygote) is then transferred to the patient's uterus with the intention of establishing a successful pregnancy.
While IVF therapy has largely replaced tubal surgery in the treatment of infertility, the presence of hydrosalpinx is a detriment to IVF success.[5] It has been recommended that prior to IVF, laparoscopic surgery should be done to either block or remove hydrosalpinges.[11]
Complementary and alternative medicine (CAM)
For fallopian tube obstruction, alternative medicine has been used as a form of fertility treatment.[12] A study of the use of alternative methods showed that only a minority of infertile couples utilize such treatments.[12] It also showed that alternative methods are more often chosen by couples who were wealthier, have not yet achieved pregnancy, or had a belief in the effectiveness of such treatments.[12] Of the study participants, 29% used a CAM modality for treatment, 22% used acupuncture, 17% used herbal therapies, and 1% using meditation.
Uterine fibroids
Definition
Uterine fibroids are noncancerous growths of the uterus that often appear during childbearing years. Also called leiomyomas (lie-o-my-O-muhs) or myomas, uterine fibroids aren't associated with an increased risk of uterine cancer and almost never develop into cancer.
Uterine fibroids develop from the smooth muscular tissue of the uterus (myometrium). A single cell divides repeatedly, eventually creating a firm, rubbery mass distinct from nearby tissue. The growth patterns of uterine fibroids vary — they may grow slowly or rapidly, or they may remain the same size. Some fibroids go through growth spurts, and some may shrink on their own. Many fibroids that have been present during pregnancy shrink or disappear after pregnancy, as the uterus goes back to a normal size.
Fibroids range in size from seedlings, undetectable by the human eye, to bulky masses that can distort and enlarge the uterus. They can be single or multiple, in extreme cases expanding the uterus so much that it reaches the rib cage.
As many as 3 out of 4 women have uterine fibroids sometime during their lives, but most are unaware of them because they often cause no symptoms. Your doctor may discover fibroids incidentally during a pelvic exam or prenatal ultrasound.
Symptoms
In women who have symptoms, the most common symptoms of uterine fibroids include:
- Heavy menstrual bleeding
- Prolonged menstrual periods — seven days or more of menstrual bleeding
- Pelvic pressure or pain
- Frequent urination
- Difficulty emptying your bladder
- Constipation
- Backache or leg pains
Rarely, a fibroid can cause acute pain when it outgrows its blood supply. Deprived of nutrients, the fibroid begins to die. Byproducts from a degenerating fibroid can seep into surrounding tissue, causing pain and, rarely, fever. A fibroid that hangs by a stalk inside or outside the uterus (pedunculated fibroid) can trigger pain by twisting on its stalk and cutting off its blood supply.
Fibroid location, size and number influence signs and symptoms:
- Submucosal fibroids. Fibroids that grow into the inner cavity of the uterus (submucosal fibroids) are more likely to cause prolonged, heavy menstrual bleeding and are sometimes a problem for women attempting pregnancy.
- Subserosal fibroids. Fibroids that project to the outside of the uterus (subserosal fibroids) can sometimes press on your bladder, causing you to experience urinary symptoms. If fibroids bulge from the back of your uterus, they occasionally can press either on your rectum, causing a pressure sensation, or on your spinal nerves, causing backache.
- Intramural fibroids. Some fibroids grow within the muscular uterine wall (intramural fibroids). If large enough, they can distort the shape of the uterus and cause prolonged, heavy periods, as well as pain and pressure.
When to see a doctor
See your doctor if you have:
- Pelvic pain that doesn't go away
- Overly heavy or painful periods
- Spotting or bleeding between periods
- Pain consistently with intercourse
- Enlarged uterus and abdomen
- Difficulty emptying your bladder
Seek prompt medical care if you have severe vaginal bleeding or sharp pelvic pain that comes on suddenly.
Causes
Doctors don't know the cause of uterine fibroids, but research and clinical experience point to these factors:
- Genetic changes. Many fibroids contain changes in genes that differ from those in normal uterine muscle cells. There's also some evidence that fibroids run in families and that identical twins are more likely to both have fibroids than nonidentical twins.
- Hormones. Estrogen and progesterone, two hormones that stimulate development of the uterine lining during eachmenstrual cycle in preparation for pregnancy, appear to promote the growth of fibroids. Fibroids contain more estrogen and progesterone receptors than normal uterine muscle cells do. Fibroids tend to shrink after menopause due to a decrease in hormone production.
- Other growth factors. Substances that help the body maintain tissues, such as insulin-like growth factor, may affect fibroid growth.
Risk factors
There are few known risk factors for uterine fibroids, other than being a woman of reproductive age. Other factors that can have an impact on fibroid development include:
- Heredity. If your mother or sister had fibroids, you're at increased risk of developing them.
- Race. Black women are more likely to have fibroids than women of other racial groups. In addition, black women have fibroids at younger ages, and they're also likely to have more or larger fibroids.
- Other factors. Onset of menstruation at an early age, having a diet higher in red meat and lower in green vegetables and fruit, and drinking alcohol, including beer, appear to increase your risk of developing fibroids.
Complications
Although uterine fibroids usually aren't dangerous, they can cause discomfort and may lead to complications such as anemia from heavy blood loss.
Pregnancy and fibroids
Fibroids usually don't interfere with conception and pregnancy. However, it's possible that fibroids could cause infertility or pregnancy loss. Submucosal fibroids may prevent implantation and growth of an embryo. In such cases, doctors often recommend removing these fibroids before attempting pregnancy or if you've had multiple miscarriages. Rarely, fibroids can distort or block your fallopian tubes, or interfere with the passage of sperm from your cervix to your fallopian tubes.
Test and diagnosis
Uterine fibroids are frequently found incidentally during a routine pelvic exam. Your doctor may feel irregularities in the shape of your uterus, suggesting the presence of fibroids. If you have symptomsof uterine fibroids, you doctor may order these tests:
- Ultrasound. If confirmation is needed, your doctor may order an ultrasound. It uses sound waves to get a picture of your uterus to confirm the diagnosis and to map and measure fibroids. A doctor or technician moves the ultrasound device (transducer) over your abdomen (transabdominal) or places it inside your vagina (transvaginal) to get images of your uterus.
- Lab tests. If you're experiencing abnormal vaginal bleeding, your doctor may order other tests to investigate potentialcauses. These might include a complete blood count (CBC) to determine if you have anemia because of chronic blood loss and other blood tests to rule out bleeding disorders or thyroid problems.
Other imaging tests
If traditional ultrasound doesn't provide enough information, your doctor may order other imaging studies, such as:
- Magnetic resonance imaging (MRI). This imaging test can show the size and location of fibroids, identify different types of tumors and help determine appropriate treatment options.
- Hysterosonography. Hysterosonography (his-tur-o-suh-NOG-ruh-fee), also called a saline infusion sonogram, uses sterile saline to expand the uterine cavity, making it easier to get images of the uterine cavity and endometrium. This test may be useful if you have heavy menstrual bleeding despite normal results from traditional ultrasound.
- Hysterosalpingography. Hysterosalpingography (his-tur-o-sal-ping-GOG-ruh-fee) uses a dye to highlight the uterine cavity and fallopian tubes on X-ray images. Your doctor may recommend it if infertility is a concern. In addition to revealing fibroids, it can help your doctor determine if your fallopian tubes are open.
- Hysteroscopy. For this, your doctor inserts a small, lighted telescope called a hysteroscope through your cervix into your uterus. Your doctor then injects saline into your uterus, expanding the uterine cavity and allowing your doctor to examine the walls of your uterus and the openings of your fallopian tubes.
Prevention
Although researchers continue to study the causes of fibroid tumors, little scientific evidence is available on how to prevent them. Preventing uterine fibroids may not be possible, but only a small percentage of these tumors require treatment.
Leukorrhea
| Leukorrhea | |
|---|---|
Leukorrhea (US) or leucorrhoea (Commonwealth) is a medical term that denotes a thick, whitish or yellowish vaginal discharge.[1]There are many causes of leukorrhea, the usual one being estrogen imbalance. The amount of discharge may increase due tovaginal infection or STDs, and also it may disappear and reappear from time to time, this discharge can keep occurring for years in which case it becomes more yellow and foul-smelling; it is usually a non-pathological symptom secondary to inflammatory conditions of vagina or cervix.
Vaginal discharge is not abnormal, and causes of change in discharge include infection, malignancy, and hormonal changes. It sometimes occurs before a girl has her first period, and is considered a sign of puberty.
Physiologic leukorrhea
It is not a major issue but is to be resolved as soon as possible. It can be a natural defense mechanism that the vagina uses to maintain its chemical balance, as well as to preserve the flexibility of the vaginal tissue. The term "physiologic leukorrhea" is used to refer to leukorrhea due to estrogen stimulation.[2]
Leukorrhea may occur normally during pregnancy. This is caused by increased bloodflow to the vagina due to increased estrogen. Female infants may have leukorrhea for a short time after birth due to their in-uterine exposure to estrogen.
Inflammatory leukorrhea
It may also result from inflammation or congestion of the vaginal mucosa. In cases where it is yellowish or gives off an odor, a doctor should be consulted since it could be a sign of several disease processes, including an organic bacterial infection or STD.
After delivery, leukorrhea accompanied by backache and foul-smelling lochia (post-partum vaginal discharge, containing blood, mucus, and placental tissue) may suggest the failure of involution (the uterus returning to pre-pregnancy size) due to infection. Investigations: wet smear, Gram stain, culture, pap smear and biopsy.
Ovarian cyst
| Ovarian cyst | |
|---|---|
| Classification and external resources | |
 |
An ovarian cyst is any collection of fluid, surrounded by a very thin wall, within an ovary.[1] Any ovarian follicle that is larger than about two centimeters is termed an ovarian cyst. Such cysts range in size from as small as a pea to larger than an orange.
Ovarian cysts affect women of all ages. They occur most often, however, during a woman's childbearing years.
Some ovarian cysts cause problems, such as bleeding and pain. Surgery may be required to remove cysts larger than 5 centimeters in diameter.
Classification
Ovarian cysts may be classified according to whether they are a variant of the normal menstrual cycle, called a functional cyst, or not.[3]
Functional
Functional cysts form as a normal part of the menstrual cycle. Such cysts may include:
- Follicular cyst, the most common type of ovarian cyst. In menstruation, a follicle containing the ovum (unfertilized egg) will rupture during ovulation. If this does not occur, a follicular cyst of more than 2.5 cm diameter may result.[3]
- Corpus luteum cysts appear after ovulation. The corpus luteum is the remnant of the follicle after the ovum has moved to the fallopian tubes. This normally degrades within 5–9 days. A corpus lutem that is more than 3 cm is defined as cystic.[3]
- Thecal cysts occur within the thecal layer of cells surrounding developing oocytes. Under the influence of excessive hCG, thecal cells may proliferate and become cystic. This is usually on both ovaries.[3]
Non-functional
Non-functional cysts may include:
- An ovary with many cysts, which may be found in normal women, or within the setting of polycystic ovary syndrome.
- Cysts caused by endometriosis, known as chocolate cysts.
- Hemorrhagic ovarian cyst
- Dermoid cyst
- Ovarian serous cystadenoma
- Ovarian mucinous cystadenoma
- Paraovarian cyst
- Cystic adenofibroma
- Borderline tumoral cysts
Signs and symptoms
Some or all of the following symptoms may be present, though it is possible not to experience any symptoms:[3]
- Abdominal pain. Dull aching pain within the abdomen or pelvis, especially on intercourse.
- Uterine bleeding. Pain during or shortly after beginning or end of menstrual period; irregular periods, or abnormal uterine bleeding or spotting.
- Fullness, heaviness, pressure, swelling, or bloating in the abdomen.
- When a cyst ruptures from the ovary, there may be sudden and sharp pain in the lower abdomen on one side.
- Change in frequency or ease of urination (such as inability to fully empty the bladder), or difficulty with bowel movements due to pressure on adjacent pelvic anatomy.
- Constitutional symptoms such as fatigue, headaches
- Nausea or vomiting
- Weight gain
Other symptoms may depend on the cause of the cysts:[3]
- Symptoms that may occur if the cause of the cysts is polycystic ovarian syndrome may include increased facial hair or body hair, acne, obesity and infertility.
- If the cause is endometriosis, then periods may be heavy, and intercourse painful.
Diagnosis
Ovarian cysts are usually diagnosed by either ultrasound or CT scan. Follow-up imaging for women of reproductive age with small simple or hemorrhagic cyst is generally not required.[4]
There are several systems for scoring of the risk of an ovarian cyst of being an ovarian cancer, including RMI (risk of malignancy index), LR2 and SR (simple rules). Sensitivities and specificities of these systems are given in tables below:[5]
| Scoring systems | Premenopausal | Postmenopausal | ||
|---|---|---|---|---|
| Sensitivity | Specificity | Sensitivity | Specificity | |
| RMI I | 44% | 95% | 79% | 90% |
| LR2 | 85% | 91% | 94% | 70% |
| SR | 93% | 83% | 93% | 76% |
Risk of malignancy index
A widely recognized method of estimating the risk of malignant ovarian cancer based on initial workup is the risk of malignancy index(RMI).[6]
It is recommended that women with an RMI score over 200 should be referred to a center with experience in ovarian cancer surgery.[7]
The RMI is calculated as follows:[7]
- RMI = ultrasound score x menopausal score x CA-125 level in U/ml.
There are two methods to determine the ultrasound score and menopausal score, with the resultant RMI being called RMI 1 and RMI 2, respectively, depending on what method is used:[7]
| Feature | RMI 1 | RMI 2 |
|---|---|---|
Ultrasound abnormalities:
|
|
|
| Menopausal score |
|
|
| CA-125 | Quantity in U/ml | Quantity in U/ml |
An RMI 2 of over 200 has been estimated to have a sensitivity of 74 to 80%, a specificity of 89 to 92% and a positive predictive value of around 80% of ovarian cancer.[7] RMI 2 is regarded as more sensitive than RMI 1.[7]
Treatment
Treatment for cysts depends on the size of the cyst and symptoms.
Pain caused by ovarian cysts may be treated with:
- Pain relievers, including acetaminophen/paracetamol (Tylenol or Panadol), nonsteroidal anti-inflammatory drugs such as ibuprofen (Motrin, Advil), or narcotic pain medicine (by prescription) may help reduce pelvic pain.[9] NSAIDs usually work best when taken at the first signs of the pain.
- A warm bath, or heating pad, or hot water bottle applied to the lower abdomen near the ovaries can relax tense muscles and relieve cramping, lessen discomfort, and stimulate circulation and healing in the ovaries.[10]
- Combined methods of hormonal contraception such as the combined oral contraceptive pill – the hormones in the pills may regulate the menstrual cycle, and prevent the formation of follicles that can turn into cysts.(American College of Obstetricians and Gynecologists, 1999c; Mayo Clinic, 2002e)[9] However, a Cochrane review in 2011 concluded oral contraceptives are of no benefit in treating already present functional cysts.[11]
Also, limiting strenuous activity may reduce the risk of cyst rupture or torsion.
Cysts that persist beyond two or three menstrual cycles, or occur in post-menopausal women, may indicate more serious disease and should be investigated throughultrasonography and laparoscopy, especially in cases where family members have had ovarian cancer. Such cysts may require surgical biopsy. Additionally, a blood test may be taken before surgery to check for elevated CA-125, a tumor marker, which is often found in increased levels in ovarian cancer, although it can also be elevated by other conditions resulting in a large number of false positives.[12]
For more serious cases where cysts are large and persisting, doctors may suggest surgery. This may involve removing the cyst, or one or both ovaries.[13] Features that may indicate the need for surgery include:[14]
- Persistent complex ovarian cysts
- Persistent cysts that are causing symptoms
- Simple ovarian cysts larger than 5-10 centimeters
- Women who are menopausal or perimenopausal
Ovarian cyst rupture
A rupture of an ovarian cyst is usually a self-limiting, and only requires expectant management and analgesics. The main symptom is abdominal pain, but can also be asymptomatic. The pain may last from a few days to several weeks.[15]
This article needs more medical references for verification or relies too heavily on primary sources. Please review the contents of the article and add the appropriate references if you can. Unsourced or poorly sourced material may be removed. (February 2014)
This article may contain inappropriate or misinterpreted citations that do not verify the text. Please help improve this article by checking for inaccuracies. (help, talk, get involved!) (February 2014)
Some or all of this article's listed sources may not be reliable. Please help this article by looking for better, more reliable sources, or by checking whether the references meet the criteria for reliable sources. Unreliable citations may be challenged or deleted. (February 2014)
Hormones are the chemical messengers in the body that travel the bloodstream to the organs and tissues. They slowly work and affect many of the body's processes over time. Endocrine glands, which are special groups of cells, make hormones.[1]
There are many endocrine glands in the body with the main ones being the pituitary gland, thyroid, thymus, adrenal glands, and the pancreas. Hormones are dominant and it only requires a small amount of them to cause significant changes throughout the body. Both men and women produce hormones in the same areas with one exception, the sexual organs. Additional male hormones are produced in the testes while women's are produced in the ovaries.
If hormone imbalance is left untreated it can result in serious medical conditions like diabetes. If the imbalance is taking place in the pituitary glands, growth disorders are possible and will require treatment of a growth hormone. It is possible that the imbalance could also cause an overproduction of growth hormones and cause medical conditions such as gigantism and acromegaly. There are approximately 6,000 endocrine disorders that result because of hormone imbalance[medical citation needed]. An imbalance of hormones is experienced at different times during life. As the body changes from childhood to adulthood, puberty is experienced by both male and females. Women will then again experience a change later in life after their childbearing years have been passed. Hormonal imbalance is defined as chemical messengers which regulate our body's systems and that are no longer functioning properly.[2][not in citation given][unreliable medical source?] This dysfunction can be an overproduction or an underproduction of specific hormones. The primary hormone that causes these changes is estrogen[medical citation needed].
This section does not cite any references or sources. Please help improve this section by adding citations to reliable sources. Unsourced material may be challenged and removed. (September 2012)
A hormonal imbalance occurs as a reaction to the elevated level of estrogen and lowered level of progesterone within a woman's body[medical citation needed]. Estrogen is naturally produced by the ovaries and is the female hormone necessary for normal sexual development. It also works to regulate the menstrual cycle to prepare and maintain the body during childbearing years. Estrogen is dominant during the follicular phase of the menstrual cycle. Progesterone is dominant during the luteal phase of the menstrual cycle by the corpus luteum, and is needed for implantation of the fertilized egg. Later in life, the ovaries begin to decrease their production of estrogen and progesterone, causing symptoms of hormone imbalance to develop. Estrogen replacement therapy is a common treatment for hormone imbalance[medical citation needed]. Frequently, only estrogen is replaced. Some health care providers, especially alternative medicine practitioners, feel it is important to supplement progesterone as well, as the balance between estrogen and progesterone is important. Estrogen dominance[medical citation needed], in which there is too much estrogen relative to progesterone, can cause infertility, PMS, menstrual problems, abdominal weight gain, and possibly increased risk of breast cancer[medical citation needed].
Causes
There are multiple causes for hormone imbalance, but the majority of cases are experienced due to estrogen dominance or increased amounts of estrogen in the body and not enough of progesterone. Common causes include birth control pills, stress, overuse of cosmetics, and non organic animal products[medical citation needed][dubious – discuss]. Other medical causes include genetics, obesity, and tumors. Other causes include lack of exercise, pregnancy, lactation, autoantibody production, and a sedentary lifestyle[medical citation needed]. Of all of these causes, environmental estrogens are the primary cause for hormone imbalance while pregnancy results in an increase in progesterone levels, thereby mitigating these symptoms[medical citation needed][dubious – discuss].
Symptoms
Some of the symptoms experienced during hormone imbalance are shared by male and female, while some are more specific to each gender. Some of the most commonly shared symptoms include fatigue, skin problems or acne, mood swings, weight problems, diminished sex drive, and no memory. If the reactions become more severe then we run into actual hormone allergy where we find a group of more serious disorders.[3] The disorders include arthritis, chronic fatigue syndrome, fibromyalgia, and anxiety attacks. The presence of urinary tract infections, increased dryness in the mouth, eyes, genitalia, or abnormal heartbeat can also be experienced[medical citation needed]. The majority of these symptoms are experienced due to menopause[medical citation needed].
In addition, hair loss, or alopecia, can be directly attributed to the irregular levels of hormones in the body[medical citation needed][dubious – discuss].
Hormones that influence the cycle of hair growth are not just those produced by the reproductive organs (e.g., testosterone produced by the testes; and estrogen and progesterone produced by the ovaries.). Hair loss can also be induced by other hormones formed in other glands, including the pituitary, thyroid, parathyroid, thymus, pineal and the adrenals. [4]
Male Hormones and Hair Loss
The hormonal process of testosterone converting to dihydrotestosterone (DHT) affects the hair growth cycle. This is because when DHT binds to the receptors, it causes the hair follicles to shrink. The shrinking of the hair follicles, referred to as the miniaturization process, produces finer, thinner and shorter hair strands. Dihydrotestosterone (DHT), a by-product of the male hormone testosterone combined with the enzyme 5-alpha reductase, is currently thought to be the primary culprit of hair loss. Though little is known about the hair follicle’s DHT receptors, it is believed that the conversion of testosterone into DHT plays a great role in the hair loss process. When the testosterone levels increase, DHT becomes more of a problem. This happens when an individual has the kind of body chemistry that is overly sensitive to even regular levels of chemicals, including hormones. Since hormones function best when they are in a delicate balance, the male hormones (androgens) do not need to be present in greater amount in order to start a problem. Such an imbalance in the hormone levels can also cause problems, including hair loss and thinning hair.[5]
Menopause
Menopause is the permanent end of menstruation and fertility, defined as occurring 12 months after a woman's last menstrual period.[6] It is the time in a woman's life when the ovaries stop producing eggs and the body doesn't produce the same amount of progesterone or estrogen. Menstruation is less frequent and eventually stops altogether. It is a biological process that is natural and is not a medical illness. Hormonal imbalance is the cause for the physical and emotional experiences associated with menopause. These symptoms include hot flushes, broken sleep patterns or insomnia, and changes in sexual response[medical citation needed]. There is no need for prevention of menopause, but there are steps that can be taken to prevent specific side effects. Regular exercise, calcium and vitamin D supplements, a low-fat diet, and controlling blood pressure and cholesterol levels are recommended[medical citation needed].
Treatment for hormone imbalance
It is important to understand all the risks and benefits of hormone replacement therapy (HRT). Patients with a history of active or past breast cancer, blood clots, liver disease, pregnancy, or endometrial cancer should talk with a physician before using an over the counter or prescription therapy[medical citation needed]. There are two main types of HRT. The first is estrogen replacement. It is available in tablet form, cream, or a patch. It is administered alone and is given in the lowest dose possible to relieve symptoms. The second type of therapy is a combination of estrogen and progesterone. It is commonly known as HRT combination therapy. These two hormones are given continuously for the shortest time possible to reduce the risk for possible side effects. Side effects of treatment include irregular spotting, breast tenderness, fluid retention, headaches, dizziness, and blood clots or stroke[medical citation needed].
Improper use of hormones
A dangerous or fatal hormone imbalance can occur in those who use anabolic steroids. While the endocrine system is developing, use of these hormones can result in a hormone imbalance that causes an increase in aggressive behavior, mood swings, or developmental disorders. Steroid use is required for some patients, but should only be administered and taken under the care of a health professional to reduce these risks[medical citation needed].
Chromosome
A chromosome is a structure of DNA, protein, and RNA found in cells. It is a single piece of coiled DNA containing many genes,regulatory elements and other nucleotide sequences. Chromosomes also contain DNA-bound proteins, which serve to package the DNA and control its functions. Chromosomal DNA encodes most or all of an organism's genetic information; some species also containplasmids or other extrachromosomal genetic elements.
Chromosomes vary widely between different organisms. The DNA molecule may be circular or linear, and can be composed of 100,000 to over 3,750,000,000[1][2] nucleotides in a long chain. Typically, eukaryotic cells (cells with nuclei) have large linear chromosomes and prokaryotic cells (cells without defined nuclei) have smaller circular chromosomes, although there are many exceptions to this rule. Cells may contain more than one type of chromosome; for example, mitochondria in most eukaryotes andchloroplasts in plants have their own small chromosomes.
In eukaryotes, nuclear chromosomes are packaged by proteins into a condensed structure called chromatin. This allows the very long DNA molecules to fit into the cell nucleus. The structure of chromosomes and chromatin varies through the cell cycle. Chromosomes are even more condensed than chromatin and are an essential unit for cellular division. Chromosomes must be replicated, divided, and passed successfully to their daughter cells so as to ensure the genetic diversity and survival of their progeny. Chromosomes may exist as either duplicated or unduplicated. Unduplicated chromosomes are single linear strands, whereas duplicated chromosomes contain two identical copies (called chromatids or sister chromatids) joined by a centromere.
Compaction of the duplicated chromosomes during mitosis and meiosis results in the classic four-arm structure (pictured to the right) if the centromere is located in the middle of the chromosome or a two-arm structure if the centromere is located near one of the ends. Chromosomal recombination plays a vital role in genetic diversity. If these structures are manipulated incorrectly, through processes known as chromosomal instability and translocation, the cell may undergo mitotic catastrophe and die, or it may unexpectedly evadeapoptosis leading to the progression of cancer.
In practice "chromosome" is a rather loosely defined term. In prokaryotes and viruses, the term genophore is more appropriate when no chromatin is present. However, a large body of work uses the term chromosome regardless of chromatin content. In prokaryotes, DNA is usually arranged as a loop, which is tightly coiled in on itself, sometimes accompanied by one or more smaller, circular DNA molecules called plasmids. These small circular genomes are also found in mitochondriaand chloroplasts, reflecting their bacterial origins. The simplest genophores are found in viruses: these DNA or RNA molecules are short linear or circular genophores that often lack structural proteins.[citation needed]
The word chromosome comes from the Greek χρῶμα (chroma, colour) and σῶμα (soma, body) due to their property of being very strongly stained by particular dyes.
History of discovery
In a series of experiments beginning in the mid-1880s, Theodor Boveri gave the definitive demonstration that chromosomes are thevectors of heredity. His two principles were the continuity of chromosomes and the individuality of chromosomes.[citation needed] It is the second of these principles that was so original.[citation needed] Wilhelm Roux suggested that each chromosome carries a different genetic load. Boveri was able to test and confirm this hypothesis. Aided by the rediscovery at the start of the 1900s ofGregor Mendel's earlier work, Boveri was able to point out the connection between the rules of inheritance and the behaviour of the chromosomes. Boveri influenced two generations of American cytologists: Edmund Beecher Wilson, Walter Sutton and Theophilus Painter were all influenced by Boveri (Wilson and Painter actually worked with him).
In his famous textbook The Cell in Development and Heredity, Wilson linked together the independent work of Boveri and Sutton (both around 1902) by naming the chromosome theory of inheritance the Boveri–Sutton chromosome theory (the names are sometimes reversed).[3] Ernst Mayr remarks that the theory was hotly contested by some famous geneticists: William Bateson,Wilhelm Johannsen, Richard Goldschmidt and T.H. Morgan, all of a rather dogmatic turn of mind. Eventually, complete proof came from chromosome maps in Morgan's own lab.[4]
The number of human chromosomes was published in 1923 by Theophilus Painter. By inspection through the microscope he counted 24 pairs which would mean 48 chromosomes. His error was copied by others and it was not until 1956 that the true number, 46, was determined by Indonesia-born cytogeneticist, Joe Hin Tjio.[5]
Prokaryotes
The prokaryotes – bacteria and archaea – typically have a single circular chromosome, but many variations exist.[6] Most bacteria's chromosome can range in size from only 160,000 base pairs in the endosymbiotic bacterium Candidatus Carsonella ruddii,[7] to 12,200,000 base pairs in the soil-dwelling bacterium Sorangium cellulosum.[8] Spirochaetesof the genus Borrelia are a notable exception to this arrangement, with bacteria such as Borrelia burgdorferi, the cause of Lyme disease, containing a single linear chromosome.[9]Some genes, known as Orphons, aren't even in a chromosome at all.
Structure in sequences
Prokaryotic chromosomes have less sequence-based structure than eukaryotes. Bacteria typically have a single point (the origin of replication) from which replication starts, whereas some archaea contain multiple replication origins.[10] The genes in prokaryotes are often organized in operons, and do not usually contain introns, unlike eukaryotes.
DNA packaging
Prokaryotes do not possess nuclei. Instead, their DNA is organized into a structure called the nucleoid.[11] The nucleoid is a distinct structure and occupies a defined region of the bacterial cell. This structure is, however, dynamic and is maintained and remodeled by the actions of a range of histone-like proteins, which associate with the bacterial chromosome.[12] In archaea, the DNA in chromosomes is even more organized, with the DNA packaged within structures similar to eukaryotic nucleosomes.[13][14]
Bacterial chromosomes tend to be tethered to the plasma membrane of the bacteria. In molecular biology application, this allows for its isolation from plasmid DNA by centrifugation of lysed bacteria and pelleting of the membranes (and the attached DNA).
Prokaryotic chromosomes and plasmids are, like eukaryotic DNA, generally supercoiled. The DNA must first be released into its relaxed state for access for transcription, regulation, and replication.
Eukaryotes
See also: Eukaryotic chromosome fine structure
Eukaryotes (cells with nuclei such as those found in plants, yeast, and animals) possess multiple large linear chromosomes contained in the cell's nucleus. Each chromosome has one centromere, with one or two arms projecting from the centromere, although, under most circumstances, these arms are not visible as such. In addition, most eukaryotes have a small circularmitochondrial genome, and some eukaryotes may have additional small circular or linear cytoplasmic chromosomes.
In the nuclear chromosomes of eukaryotes, the uncondensed DNA exists in a semi-ordered structure, where it is wrapped aroundhistones (structural proteins), forming a composite material called chromatin.
Chromatin
Main article: Chromatin
Chromatin is the complex of DNA and protein found in the eukaryotic nucleus, which packages chromosomes. The structure of chromatin varies significantly between different stages of the cell cycle, according to the requirements of the DNA.
Interphase chromatin
During interphase (the period of the cell cycle where the cell is not dividing), two types of chromatin can be distinguished:
- Euchromatin, which consists of DNA that is active, e.g., being expressed as protein.
- Heterochromatin, which consists of mostly inactive DNA. It seems to serve structural purposes during the chromosomal stages. Heterochromatin can be further distinguished into two types:
- Constitutive heterochromatin, which is never expressed. It is located around the centromere and usually contains repetitive sequences.
- Facultative heterochromatin, which is sometimes expressed.
Metaphase chromatin and division
In the early stages of mitosis or meiosis (cell division), the chromatin strands become more and more condensed. They cease to function as accessible genetic material (transcription stops) and become a compact transportable form. This compact form makes the individual chromosomes visible, and they form the classic four arm structure, a pair of sister chromatids attached to each other at the centromere. The shorter arms are called p arms (from the French petit, small) and the longer arms are called q arms (q follows p in the Latin alphabet; q-g "grande"; alternatively it is sometimes said q is short for queue meaning tail in French[15]). This is the only natural context in which individual chromosomes are visible with an optical microscope.
During mitosis, microtubules grow from centrosomes located at opposite ends of the cell and also attach to the centromere at specialized structures called kinetochores, one of which is present on each sister chromatid. A special DNA base sequence in the region of the kinetochores provides, along with special proteins, longer-lasting attachment in this region. The microtubules then pull the chromatids apart toward the centrosomes, so that each daughter cell inherits one set of chromatids. Once the cells have divided, the chromatids are uncoiled and DNA can again be transcribed. In spite of their appearance, chromosomes are structurally highly condensed, which enables these giant DNA structures to be contained within a cell nucleus (Fig. 2).
Human chromosomes
Chromosomes in humans can be divided into two types: autosomes and sex chromosomes. Certain genetic traits are linked to a person's sex and are passed on through the sex chromosomes. The autosomes contain the rest of the genetic hereditary information. All act in the same way during cell division. Human cells have 23 pairs of chromosomes (22 pairs of autosomes and one pair of sex chromosomes), giving a total of 46 per cell. In addition to these, human cells have many hundreds of copies of the mitochondrial genome.Sequencing of the human genome has provided a great deal of information about each of the chromosomes. Below is a table compiling statistics for the chromosomes, based on the Sanger Institute's human genome information in the Vertebrate Genome Annotation (VEGA) database.[16] Number of genes is an estimate as it is in part based on gene predictions. Total chromosome length is an estimate as well, based on the estimated size of unsequenced heterochromatin regions.
| Chromosome | Genes | Total base pairs | Sequenced base pairs[17] | Cumulative (%) |
|---|---|---|---|---|
| 1 | 4,220 | 247,199,719 | 224,999,719 | 7.9 |
| 2 | 1,491 | 242,751,149 | 237,712,649 | 16.2 |
| 3 | 1,550 | 199,446,827 | 194,704,827 | 23.0 |
| 4 | 446 | 191,263,063 | 187,297,063 | 29.6 |
| 5 | 609 | 180,837,866 | 177,702,766 | 35.8 |
| 6 | 2,281 | 170,896,993 | 167,273,993 | 41.6 |
| 7 | 2,135 | 158,821,424 | 154,952,424 | 47.1 |
| 8 | 1,106 | 146,274,826 | 142,612,826 | 52.0 |
| 9 | 1,920 | 140,442,298 | 120,312,298 | 56.3 |
| 10 | 1,793 | 135,374,737 | 131,624,737 | 60.9 |
| 11 | 379 | 134,452,384 | 131,130,853 | 65.4 |
| 12 | 1,430 | 132,289,534 | 130,303,534 | 70.0 |
| 13 | 924 | 114,127,980 | 95,559,980 | 73.4 |
| 14 | 1,347 | 106,360,585 | 88,290,585 | 76.4 |
| 15 | 921 | 100,338,915 | 81,341,915 | 79.3 |
| 16 | 909 | 88,822,254 | 78,884,754 | 82.0 |
| 17 | 1,672 | 78,654,742 | 77,800,220 | 84.8 |
| 18 | 519 | 76,117,153 | 74,656,155 | 87.4 |
| 19 | 1,555 | 63,806,651 | 55,785,651 | 89.3 |
| 20 | 1,008 | 62,435,965 | 59,505,254 | 91.4 |
| 21 | 578 | 46,944,323 | 34,171,998 | 92.6 |
| 22 | 1,092 | 49,528,953 | 34,893,953 | 93.8 |
| X (sex chromosome) | 1,846 | 154,913,754 | 151,058,754 | 99.1 |
| Y (sex chromosome) | 454 | 57,741,652 | 25,121,652 | 100.0 |
| Total | 32,185 | 3,079,843,747 | 2,857,698,560 | 100.0 |
Number of chromosomes in various organisms
Main article: List of number of chromosomes of various organisms
Eukaryotes
These tables give the total number of chromosomes (including sex chromosomes) in a cell nucleus. For example, human cells are diploid and have 22 different types of autosome, each present as two copies, and two sex chromosomes. This gives 46 chromosomes in total. Other organisms have more than two copies of their chromosomes, such as bread wheat, which is hexaploid and has six copies of seven different chromosomes – 42 chromosomes in total.
|
|
|
Normal members of a particular eukaryotic species all have the same number of nuclear chromosomes (see the table). Other eukaryotic chromosomes, i.e., mitochondrial and plasmid-like small chromosomes, are much more variable in number, and there may be thousands of copies per cell.
Asexually reproducing species have one set of chromosomes, which are the same in all body cells. However, asexual species can be either haploid or diploid.
Sexually reproducing species have somatic cells (body cells), which are diploid [2n] having two sets of chromosomes (23 pairs in humans with one set of 23 chromosomes from each parent), one set from the mother and one from the father. Gametes, reproductive cells, are haploid [n]: They have one set of chromosomes. Gametes are produced by meiosis of a diploid germ line cell. During meiosis, the matching chromosomes of father and mother can exchange small parts of themselves (crossover), and thus create new chromosomes that are not inherited solely from either parent. When a male and a female gamete merge (fertilization), a new diploid organism is formed.
Some animal and plant species are polyploid [Xn]: They have more than two sets of homologous chromosomes. Plants important in agriculture such as tobacco or wheat are often polyploid, compared to their ancestral species. Wheat has a haploid number of seven chromosomes, still seen in some cultivars as well as the wild progenitors. The more-common pasta and bread wheats are polyploid, having 28 (tetraploid) and 42 (hexaploid) chromosomes, compared to the 14 (diploid) chromosomes in the wild wheat.[43]
Prokaryotes
Prokaryote species generally have one copy of each major chromosome, but most cells can easily survive with multiple copies.[44] For example, Buchnera, a symbiont of aphids has multiple copies of its chromosome, ranging from 10–400 copies per cell.[45] However, in some large bacteria, such as Epulopiscium fishelsoni up to 100,000 copies of the chromosome can be present.[46] Plasmids and plasmid-like small chromosomes are, as in eukaryotes, highly variable in copy number. The number of plasmids in the cell is almost entirely determined by the rate of division of the plasmid – fast division causes high copy number.
Karyotype
Main article: Karyotype
In general, the karyotype is the characteristic chromosome complement of a eukaryote species.[47] The preparation and study of karyotypes is part of cytogenetics.
Although the replication and transcription of DNA is highly standardized in eukaryotes, the same cannot be said for their karyotypes, which are often highly variable. There may be variation between species in chromosome number and in detailed organization. In some cases, there is significant variation within species. Often there is:
- 1. variation between the two sexes
- 2. variation between the germ-line and soma (between gametes and the rest of the body)
- 3. variation between members of a population, due to balanced genetic polymorphism
- 4. geographical variation between races
- 5. mosaics or otherwise abnormal individuals.
Also, variation in karyotype may occur during development from the fertilised egg.
The technique of determining the karyotype is usually called karyotyping. Cells can be locked part-way through division (in metaphase) in vitro (in a reaction vial) with colchicine. These cells are then stained, photographed, and arranged into a karyogram, with the set of chromosomes arranged, autosomes in order of length, and sex chromosomes (here X/Y) at the end: Fig. 3.
Like many sexually reproducing species, humans have special gonosomes (sex chromosomes, in contrast to autosomes). These are XX in females and XY in males.
Historical note
Investigation into the human karyotype took many years to settle the most basic question: How many chromosomes does a normal diploid human cell contain? In 1912, Hans von Winiwarter reported 47 chromosomes in spermatogonia and 48 in oogonia, concluding an XX/XO sex determination mechanism.[48] Painter in 1922 was not certain whether the diploid number of man is 46 or 48, at first favouring 46.[49] He revised his opinion later from 46 to 48, and he correctly insisted on humans having an XX/XY system.[50]
New techniques were needed to definitively solve the problem:
- Using cells in culture
- Arresting mitosis in metaphase by a solution of colchicine
- Pretreating cells in a hypotonic solution 0.075 m KCl, which swells them and spreads the chromosomes
- Squashing the preparation on the slide forcing the chromosomes into a single plane
- Cutting up a photomicrograph and arranging the result into an indisputable karyogram.
It took until 1954 before the human diploid number was confirmed as 46.[51][52] Considering the techniques of Winiwarter and Painter, their results were quite remarkable.[53]Chimpanzees (the closest living relatives to modern humans) have 48 chromosomes (as well as the other great apes: in humans two chromosomes fused to form chromosome 2).
Aberrations
Chromosomal aberrations are disruptions in the normal chromosomal content of a cell and are a major cause of genetic conditions in humans, such as Down syndrome, although most aberrations have little to no effect. Some chromosome abnormalities do not cause disease in carriers, such as translocations, or chromosomal inversions, although they may lead to a higher chance of bearing a child with a chromosome disorder. Abnormal numbers of chromosomes or chromosome sets, called aneuploidy, may be lethal or may give rise to genetic disorders. Genetic counseling is offered for families that may carry a chromosome rearrangement.
The gain or loss of DNA from chromosomes can lead to a variety of genetic disorders. Human examples include:
- Cri du chat, which is caused by the deletion of part of the short arm of chromosome 5. "Cri du chat" means "cry of the cat" in French; the condition was so-named because affected babies make high-pitched cries that sound like those of a cat. Affected individuals have wide-set eyes, a small head and jaw, moderate to severe mental health problems, and are very short.
- Down syndrome, the most common trisomy, usually caused by an extra copy of chromosome 21 (trisomy 21). Characteristics include decreased muscle tone, stockier build, asymmetrical skull, slanting eyes and mild to moderate developmental disability.[54]
- Edwards syndrome, or trisomy-18, the second most common trisomy.[citation needed] Symptoms include motor retardation, developmental disability and numerous congenital anomalies causing serious health problems. Ninety percent of those affected die in infancy. They have characteristic clenched hands and overlapping fingers.
- Isodicentric 15, also called idic(15), partial tetrasomy 15q, or inverted duplication 15 (inv dup 15).
- Jacobsen syndrome, which is very rare. It is also called the terminal 11q deletion disorder.[55] Those affected have normal intelligence or mild developmental disability, with poor expressive language skills. Most have a bleeding disorder called Paris-Trousseau syndrome.
- Klinefelter syndrome (XXY). Men with Klinefelter syndrome are usually sterile, and tend to be taller and have longer arms and legs than their peers. Boys with the syndrome are often shy and quiet, and have a higher incidence of speech delay and dyslexia. Without testosterone treatment, some may develop gynecomastia during puberty.
- Patau Syndrome, also called D-Syndrome or trisomy-13. Symptoms are somewhat similar to those of trisomy-18, without the characteristic folded hand.
- Small supernumerary marker chromosome. This means there is an extra, abnormal chromosome. Features depend on the origin of the extra genetic material. Cat-eye syndrome and isodicentric chromosome 15 syndrome (or Idic15) are both caused by a supernumerary marker chromosome, as is Pallister-Killian syndrome.
- Triple-X syndrome (XXX). XXX girls tend to be tall and thin and have a higher incidence of dyslexia.
- Turner syndrome (X instead of XX or XY). In Turner syndrome, female sexual characteristics are present but underdeveloped. Females with Turner syndrome often have a short stature, low hairline, abnormal eye features and bone development and a "caved-in" appearance to the chest.
- XYY syndrome. XYY boys are usually taller than their siblings. Like XXY boys and XXX girls, they are more likely to have learning difficulties.
- Wolf-Hirschhorn syndrome, which is caused by partial deletion of the short arm of chromosome 4. It is characterized by growth retardation, delayed motor skills development, "Greek Helmet" facial features, and mild to profound mental health problems.