Sepsis
| Sepsis | |
|---|---|
| Classification and external resources | |

Blood culture bottles: orange label for anaerobes, blue label for aerobes, and yellow label for pediatrics
| |
Sepsis (/ˈsɛpsɨs/; Greek σῆψις, putrefaction and decay) is a potentially fatal whole-body inflammation (a systemic inflammatory response syndrome or SIRS) caused by severe infection.[1][2] Sepsis can continue even after the infection that caused it is gone. Severe sepsis is sepsis complicated by organ dysfunction. Septic shock is sepsis complicated by a high lactate level or by shock that does not improve after fluid resuscitation.[3] Bacteremia is the presence of viable bacteria in the blood. The term septicemia, the presence of microorganisms or their toxins in the blood, is no longer used by the consensus committee.[2]
Sepsis causes millions of deaths globally each year.[4]
Sepsis is caused by the immune system's response to a serious infection, most commonly bacteria, but also fungi, viruses, andparasites in the blood, urinary tract, lungs, skin, or other tissues. Sepsis can be thought of as falling within a continuum from infection to multiple organ dysfunction syndrome.[5]
Common symptoms of sepsis include those related to a specific infection, but usually accompanied by high fevers, hot, flushed skin,elevated heart rate, hyperventilation, altered mental status, swelling, and low blood pressure. In the very young and elderly, or in people with weakened immune systems, the pattern of symptoms may be atypical, with hypothermia and without an easily localizable infection.[6][7]
Sepsis is usually treated with intravenous fluids and antibiotics. If fluid replacement is not sufficient to maintain blood pressure,vasopressors can be used. Mechanical ventilation and dialysis may be needed to support the function of the lungs and kidneys, respectively. To guide therapy, a central venous catheter and an arterial catheter may be placed; measurement of other hemodynamic variables (such as cardiac output, mixed venous oxygen saturation or stroke volume variation) may also be used. Sepsis patients require preventive measures for deep vein thrombosis, stress ulcers and pressure ulcers, unless other conditions prevent this. Some might benefit from tight control of blood sugar levels with insulin (targeting stress hyperglycemia).[4] The use ofcorticosteroids is controversial.[8] Activated drotrecogin alfa (recombinant activated protein C), originally marketed for severe sepsis, has not been found to be helpful, and has recently been withdrawn from sale.[9]
Signs and symptoms
In addition to symptoms related to the provoking infection, sepsis is frequently associated with either fever or hypothermia, rapid breathing,elevated heart rate, confusion, and edema.[10] Early signs are elevated heart rate, decreased urination, and elevated blood sugar, while signs of established sepsis are confusion, metabolic acidosis with compensatory respiratory alkalosis (which can manifest as faster breathing), low blood pressure, decreased systemic vascular resistance, higher cardiac output, and dysfunctions of blood coagulation.[11]
Sepsis may also lead to a drop in blood pressure, resulting in shock. This may result in light-headedness. Bruising or intense bleeding may also occur.
Cause
The most common primary sources of infection resulting in sepsis are the lungs, the abdomen, and the urinary tract.[12] Typically, 50% of all sepsis cases start as an infection in the lungs. No source is found in one third of cases.[12]
The infectious agents are usually bacteria but can also be fungi and viruses.[12] While gram-negative bacteria were previously the most common cause of sepsis, in the last decade, gram-positive bacteria, most commonly staphylococci, are thought to cause more than 50% of cases of sepsis.[13]
Diagnosis
| Finding | Value |
|---|---|
| Temperature | <36 °C (96.8 °F) or >38 °C (100.4 °F) |
| Heart rate | >90/min |
| Respiratory rate | >20/min or PaCO2<32 mmHg (4.3 kPa) |
| WBC | <4x109/L (<4000/mm³), >12x109/L (>12,000/mm³), or 10% bands |
Prompt diagnosis is crucial to the management of sepsis, as initiation of early-goal-directed therapy is key to reducing mortality from severe sepsis.[15]
Within the first three hours of suspected sepsis, diagnostic studies should include measurement of serum lactate, obtaining appropriate cultures before initiation of antimicrobial treatment, so long as this does not delay antimicrobial treatment by more than 45 minutes.[15] To identify the causative organism(s), at least two sets of blood cultures(aerobic and anaerobic bottles) should be obtained, with at least one drawn percutaneouslyand one drawn through each vascular access device (such as an IV catheter) in place more than 48 hours.[15] If other sources are suspected, cultures of these sources, such as urine, cerebrospinal fluid, wounds, or respiratory secretions, should be obtained as well, so long as this does not delay antimicrobial treatment.[15]
Within six hours, if there is persistent hypotension despite initial fluid resuscitation of 30 ml/kg, or if initial lactate is ≥ 4 mmol/L (36 mg/dL), central venous pressure and central venous oxygen saturation should be measured.[15] Lactate should be re-measured if the initial lactate was elevated.[15]
Within twelve hours, it is essential to diagnose or exclude any source of infection that would require emergent source control, such as necrotizing soft tissue infection, peritonitis,cholangitis, intestinal infarction.[15]
Definitions
According to the American College of Chest Physicians and the Society of Critical Care Medicine, there are different levels of sepsis:[2]
- Systemic inflammatory response syndrome (SIRS) is the presence of two or more of the following: abnormal body temperature, heart rate, respiratory rate or blood gas, andwhite blood cell count.
- Sepsis is defined as SIRS in response to an infectious process.[16]
- Severe sepsis is defined as sepsis with sepsis-induced organ dysfunction or tissue hypoperfusion (manifesting as hypotension, elevated lactate, or decreased urine output).[17]
- Septic shock is severe sepsis plus persistently low blood pressure following the administration of intravenous fluids.[4]
Infection
Infection can be suspected or proven (by culture, stain, or polymerase chain reaction (PCR)), or a clinical syndrome pathognomonic for infection. Specific evidence for infection includes WBCs in normally sterile fluid (such as urine or cerebrospinal fluid (CSF)); evidence of a perforated viscus (free air on abdominal x-ray or CT scan; signs of acute peritonitis); abnormal chest x-ray (CXR) consistent with pneumonia (with focal opacification); or petechiae, purpura, or purpura fulminans.
End-organ dysfunction
Examples of end-organ dysfunction include the following:[18]
- Lungs:acute respiratory distress syndrome (ARDS) (PaO2/FiO2 < 300)[note 1]
- Brain: encephalopathy symptoms: agitation, confusion, coma; cause: ischemia, hemorrhage, microthrombi, microabscesses, multifocal necrotizing leukoencephalopathy
- Liver: disruption of protein synthetic function: manifests acutely as progressive coagulopathy due to inability to synthesize clotting factors, disruption of metabolic functions: manifests as cessation of bilirubin metabolism, resulting in elevated unconjugated serum bilirubin levels
- Kidney: oliguria and anuria, electrolyte abnormalities, volume overload
- Heart: systolic and diastolic heart failure, likely due to cytokines that depress myocyte function, cellular damage, manifest as a troponin leak (although not necessarily ischemic in nature)
More specific definitions of end-organ dysfunction exist for SIRS in pediatrics.[21]
- Cardiovascular dysfunction (after fluid resuscitation with at least 40 ml/kg of crystalloid)
- hypotension with blood pressure < 5th percentile for age or systolic blood pressure < 2 standard deviations below normal for age, OR
- vasopressor requirement, OR
- two of the following criteria:
- unexplained metabolic acidosis with base deficit > 5 mEq/L
- lactic acidosis: serum lactate 2 times the upper limit of normal
- oliguria (urine output < 0.5 ml/kg/hr)
- prolonged capillary refill > 5 seconds
- core to peripheral temperature difference > 3 °C
- Respiratory dysfunction (in the absence of cyanotic heart disease or known chronic lung disease)
- the ratio of the arterial partial-pressure of oxygen to the fraction of oxygen in the gases inspired (PaO2/FiO2) < 300 (the definition of acute lung injury), OR
- arterial partial-pressure of carbon dioxide (PaCO2) > 65 torr (20 mmHg) over baseline PaCO2 (evidence of hypercapnic respiratory failure), OR
- supplemental oxygen requirement of greater than FiO2 0.5 to maintain oxygen saturation ≥ 92%
- Neurologic dysfunction
- Glasgow Coma Score (GCS) ≤ 11, OR
- altered mental status with drop in GCS of 3 or more points in a patient with developmental delay/intellectual disability
- Hematologic dysfunction
- platelet count < 80,000/mm3 or 50% drop from maximum in chronically thrombocytopenic patients, OR
- international normalized ratio (INR) > 2
- Disseminated intravascular coagulation
- Renal dysfunction
- serum creatinine ≥ 2 times the upper limit of normal for age or 2-fold increase in baseline creatinine in patients with chronic kidney disease
- Hepatic dysfunction (only applicable to infants > 1 month)
- total serum bilirubin ≥ 4 mg/dl, OR
- alanine aminotransferase (ALT) ≥ 2 times the upper limit of normal
Consensus definitions, however, continue to evolve, with the latest expanding the list of signs and symptoms of sepsis to reflect clinical bedside experience.[1]
Differential diagnosis
The differential diagnosis for sepsis is broad and includes those conditions that can cause the systemic signs of SIRS: alcohol withdrawal, pulmonary embolus, thyrotoxicosis,anaphylaxis, adrenal insufficiency, and neurogenic shock.[11]
Neonatal sepsis
In common clinical usage, neonatal sepsis specifically refers to the presence of a bacterial blood stream infection (BSI), such as meningitis, pneumonia, pyelonephritis, orgastroenteritis, in the setting of fever. Criteria with regards to hemodynamic compromise or respiratory failure are not useful because these symptoms often do not arise in neonates until death is imminent and unpreventable.
Pathophysiology
Sepsis is caused by a combination of factors related to the invading organism(s) and the host (predisposing illnesses, genetics, and immune system).[22]
Microbial factors
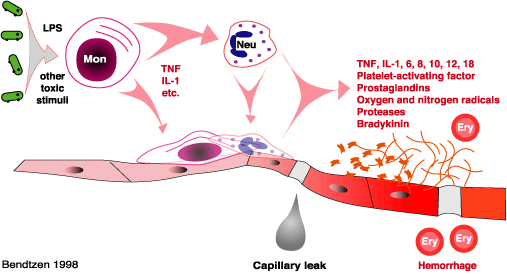
A bacteria's capsule (for example, in certain strains of Streptococcus pneumoniae), can allow it to evade phagocytosis, while pili of some strains of E. Coli can allow this bacterium to adhere to the epithelium of the kidneys.[22] Sepsis caused by gram negative bacteria is thought to be largely due to the host's response to lipopolysaccharides, also called LPS or endotoxin, in the cell wall, while gram positive bacteria are more likely to cause sepsis by their release of exotoxins.[22] Some exotoxins can quickly lead to a rapid release of cytokines by acting as superantigens, which can simultaneously bind MHC and the T-cell receptor.[22]
There are number of microbial factors which can cause the typical septic inflammatory cascade. An invading pathogen is recognised by its pathogen-associated molecular pattern(PAMP). Examples of PAMPs are lipopolysaccharides in Gram-negative bacteria, flagellin in Gram-negative bacteria, muramyl dipeptide in the peptidoglycan cell wall of a Gram-positive bacteria and CpG bacterial DNA. These PAMPs are recognised by the innate immune system's pattern recognition receptors (PRR). These receptors can be membrane-bound or cytosolic.[23] There are four families of PRRs: the toll-like receptors, the C-type lectin receptors, the nucleotide oligemerization domain-like receptors and the RigI-helicases. The association of a PAMP and a PRR will invariably cause a series of intracellular signalling cascades. Consequentially transcription factors like nuclear factor-kappa Band activating protein-1 will up regulate the expression of pro-inflammatory and anti-inflammatory cytokines.[24]
Host factors
Severe sepsis occurs when sepsis leads to organ dysfunction, such as pulmonary dysfunction, coagulation or other blood abnormalities, decreased urine production, or altered mental status. If the organ dysfunction of severe sepsis is associated with low blood pressure (hypotension), or insufficient blood flow (hypoperfusion) to one or more organs (causing, for example, lactic acidosis), this is septic shock.
Sepsis can lead to multiple organ dysfunction syndrome (MODS) (formerly known as multiple organ failure), and death. Organ dysfunction results from local changes in blood flow, from sepsis-induced hypotension (< 90 mmHg or a reduction of ≥ 40 mmHg from baseline) and from diffuse intravascular coagulation, among other things. One of the factors which appears to promote the development of MODS is the cytokine-induced abnormalities to microcirculation which has been observed as microvascular thrombosis within septic patients.[citation needed]
Endotoxins produced from bacteria and cytokines, particularly TNF, IL-1 and IL-6, can activate the procoagulation factors in the endothelium, leading to endothelial damage. This damaged endothelial surface inhibits anticoagulant properties as well as increases antifibrinolysis, which can lead to intravascular clotting, microvascular thrombosis and multiple organ failure.[25]
Bacteremia is the presence of viable bacteria in the bloodstream. Likewise, the terms viremia and fungemia simply refer to viruses and fungi in the bloodstream. These terms say nothing about the consequences this has on the body. For example, bacteria can be introduced into the bloodstream during toothbrushing.[26] This form of bacteremia almost never causes problems in normal individuals. However, bacteremia associated with certain dental procedures can cause bacterial infection of the heart valves (known asendocarditis) in high-risk patients.[27] Conversely, a systemic inflammatory response syndrome can occur in patients without the presence of infection, for example in those withburns, polytrauma, or the initial state in pancreatitis and chemical pneumonitis.[2]
Management
The therapy of sepsis rests on intravenous fluids, antibiotics, surgical drainage of infected fluid collections, and appropriate support for organ dysfunction. This may includehemodialysis in kidney failure, mechanical ventilation in pulmonary dysfunction, transfusion of blood products, and drug and fluid therapy for circulatory failure. Ensuring adequate nutrition—preferably by enteral feeding, but if necessary by parenteral nutrition—is important during prolonged illness.
In those with high blood sugar levels, insulin to bring it down to 7.8-10 mmol/L (140–180 mg/dL) is recommended with lower levels potentially worsening outcomes.[28] Medication to prevent deep vein thrombosis and gastric ulcers may also be used.[4]
Antibiotics
In severe sepsis, broad spectrum antibiotics are recommended within 1 hour of making the diagnosis.[4] For every hour delay in the administration there is an associated 6% rise in mortality.[16] Antibiotic regimens should be reassessed daily and narrowed if appropriate.[15] Duration of treatment is typically 7–10 days with the type of antibiotic used directed by the results of cultures.[4]
Early goal directed therapy
Early goal directed therapy (EGDT) is an approach to the management of severe sepsis during the initial 6 hours after diagnosis.[4] A step-wise approach should be used, with the physiologic goal of optimizing cardiac preload, afterload, and contractility.[29] It has been found to reduce mortality in those with sepsis.[30]
Urine output is also monitored, with a minimum goal of 0.5 ml/kg/h. In the original trial, mortality was cut from 46.5% to 30.5%.[29] An appropriate decrease in serum lactatehowever may be equivalent to SvO2 and easier to obtain.[31]
Intravenous fluids
In EGDT, fluids are titrated in response to heart rate, blood pressure, and urine output; restoring large fluid deficits can require 6 to 10L of crystalloids.[32] In cases where a central venous catheter is used to measure blood pressures dynamically, fluids should be administered until the central venous pressure (CVP) reaches 8–12 cm of water (or 10–15 cm of water in mechanically ventilated patients). Once these goals are met, the mixed venous oxygen saturation (SvO2), i.e., the oxygen saturation of venous blood as it returns to the heart as measured at the vena cava, is optimized. If the SvO2 is less than 70%, blood is given to reach a hemoglobin of 10 g/dl and then inotropes are added until the SvO2 is optimized.[32]
Vasopressors
Once the person has been sufficiently fluid resuscitated but the mean arterial pressure is not greater than 65 mmHg vasopressors are recommended.[4] While current recommendations suggest either norepinephrine (noradrenaline) or dopamine,[4] the former appears safer.[36][37] If a single pressor is insufficient to raise the blood pressure,epinephrine (adrenaline) may be added.[4]
Ventilation
Tracheal intubation and mechanical ventilation may be performed to reduce oxygen demand if the SvO2 remains low despite optimization of hemodynamics.[citation needed]Etomidate is not recommended as a medication to help with intubation in this situation due to concerns it may lead to poor adrenal function and an increased risk of death.[38][39]
Steroids
The use of steroids in sepsis is controversial.[8] During critical illness, a state of adrenal insufficiency and tissue resistance to corticosteroids may occur. This has been termedcritical illness–related corticosteroid insufficiency.[40] Treatment with corticosteroids might be most beneficial in those with septic shock and early severe acute respiratory distress syndrome (ARDS), whereas its role in others such as those with pancreatitis or severe pneumonia is unclear.[40] However, the exact way of determining corticosteroid insufficiency remains problematic. It should be suspected in those poorly responding to resuscitation with fluids and vasopressors. ACTH stimulation testing is not recommended to confirm the diagnosis.[40] The method of cessation of glucocorticoid drugs is variable, and it is unclear whether they should be weaned or simply stopped abruptly.
Activated protein C
Recombinant activated protein C (drotrecogin alpha) was originally introduced for severe sepsis (as identified by a high APACHE II score), where it was thought to confer a survival benefit.[4] However, subsequent studies showed that it increased adverse events and did not decrease mortality.[9] It was removed from sale in 2011.[9]
Neonates
Neonatal sepsis is difficult to diagnose. Newborns may be relatively asymptomatic until hemodynamic and respiratory collapse is imminent. If there is even a remote suspicion of sepsis, they are frequently treated with antibiotics empirically until cultures are sufficiently proven to be negative.
Prognosis
Approximately 20–35% of people with severe sepsis and 30–70% of people with septic shock die.[41] Lactate is a useful method of determining prognosis with those who have a level greater than 4 mmol/L having a mortality of 40% and those with a level of less than 2 mmol/L have a mortality of less than 15%.[16]
There are a number of prognostic stratification systems such as APACHE II and Mortality in Emergency Department Sepsis. APACHE II factors in the person's age, underlying condition, and various physiologic variables can yield estimates of the risk of dying of severe sepsis. Of the individual covariates, the severity of underlying disease most strongly influences the risk of death. Septic shock is also a strong predictor of short- and long-term mortality. Case-fatality rates are similar for culture-positive and culture-negative severe sepsis. The Mortality in Emergency Department Sepsis (MEDS) score is simpler and useful in the emergency department environment.[42]
Some people may experience severe long-term cognitive decline following an episode of severe sepsis, but the absence of baseline neuropsychological data in most sepsis patients makes the incidence of this difficult to quantify or to study.[43]
Epidemiology
Sepsis causes millions of deaths globally each year.[4] In the United States sepsis affects approximately 3 in 1,000 people,[16] and severe sepsis contributes to more than 200,000 deaths per year.[44]
Sepsis occurs in 1-2% of all hospitalizations and accounts for as much as 25% of ICU bed utilization. Due to it rarely being reported as a primary diagnosis (often being a complication of cancer or other illness), the incidence, mortality, and morbidity rates of sepsis are likely underestimated.[22] A study by the Agency for Healthcare Research and Quality (AHRQ) of selected States found that there were approximately 651 hospital stays per 100,000 population with a septicemia diagnosis in 2010.[45] It is the second-leading cause of death in non-coronary intensive care unit (ICU) patients, and the tenth-most-common cause of death overall (the first being heart disease).[46] Children under 12 months and elderly have the highest incidence of severe sepsis.[22] Among U.S. patients who had multiple septicemia hospital admissions in 2010, those who were discharged to a skilled nursing facility or long term care following the initial hospitalization were more likely to be readmitted than those discharged to another form of care.[45] A study of 18 U.S. States found that, amongst Medicare patients in 2011, septicemia was the second most common principle reason for readmission within 30 days.[47]
History
The term Σήψις[48] (sepsis) was introduced by Hippocrates in the fourth century BC, and it meant the process of decay or decomposition of organic matter.[49] In the eleventh century, Avicenna used the term "blood rot" for diseases linked to severe purulent process. Though severe systemic toxicity was observed prior, it was only in the 19th century that a specific term – sepsis – was coined for this condition.
By the end of the 19th century, it was widely believed that microbes produced substances that could injure the mammalian host and that soluble toxins released during infection caused the fever and shock that were commonplace during severe infections. Pfeiffer coined the term endotoxin at the beginning of the 20th century to denote the pyrogenic principle associated with Vibrio cholerae. It was soon realised that endotoxins were expressed by most and perhaps all Gram-negative organisms. The lipopolysaccharide character of enteric endotoxins was elucidated in 1944 by Shear.[50] The molecular character of this material was determined by Luderitz et al in 1973.[51]
It was discovered in 1965 that a strain of C3H/HeJ mice were immune to the endotoxin-induced shock.[52] The genetic locus for this effect was dubbed Lps. These mice were also found to be hypersusceptible to infection by Gram-negative bacteria.[53] These observations were finally linked in 1998 by the discovery of the Toll-like receptor gene 4 (TLR 4).[54] Genetic mapping work, performed over a period of five years, showed that TLR4 was the sole candidate locus within the Lps critical region; this strongly implied that a mutation within TLR4 must account for the lipopolysaccharide resistance phenotype. The defect in the TLR4 gene that led to the endotoxin resistant phenotype was discovered to be due to a mutation in the cytoplasmic domain.[54]
Society and culture
Economics
Septicemia was the most expensive condition seen in U.S. hospital stays in 2011, at an aggregate cost of $20.3 billion for nearly 1.1 million hospitalizations.[55] Costs for septicemia stays more than quadrupled since 1997 with an 11.5 percent annual increase.[56] By payer, it was the most costly condition billed to Medicare, the second-most costly billed to Medicaid and the uninsured, and the fourth-most costly billed to private insurance.[55]
Education
A large international collaboration entitled the "Surviving Sepsis Campaign" was established in 2002[57] to educate people about sepsis and to improve patient outcomes with sepsis. The Campaign has published an evidence-based review of management strategies for severe sepsis, with the aim to publish a complete set of guidelines in subsequent years.[4]
















.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
























No comments:
Post a Comment