Abdominal aortic aneurysm
| Abdominal aortic aneurysm | |
|---|---|
| Classification and external resources | |

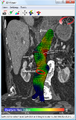
CT reconstruction image of an abdominal aortic aneurysm
| |
| ICD-10 | I71.3, I71.4 |
| ICD-9 | 441.3, 441.4 |
| OMIM | 100070 |
| DiseasesDB | 792 |
| MedlinePlus | 000162 |
| eMedicine | med/3443 emerg/27 radio/1 |
| MeSH | D017544 |
Abdominal aortic aneurysm (also known as AAA,[1] pronounced "triple-a") is a localizeddilatation (ballooning) of the abdominal aorta exceeding the normal diameter by more than 50 percent, and is the most common form of aortic aneurysm. Approximately 90 percent of abdominal aortic aneurysms occur infrarenally (below the kidneys), but they can also occurpararenally (at the level of the kidneys) or suprarenally (above the kidneys). Such aneurysms can extend to include one or both of the iliac arteries in the pelvis.
Abdominal aortic aneurysms occur most commonly in individuals between 65 and 75 years old and are more common among men and smokers. They tend to cause no symptoms, although occasionally they cause pain in the abdomen and back (due to pressure on surrounding tissues) or in the legs (due to disturbed blood flow). The major complication of abdominal aortic aneurysms is rupture, which is life-threatening, as large amounts of blood spill into the abdominal cavity, and can lead to death within minutes.[2] Mortality of rupture repair in the hospital is 60% to 90%.
Treatment is usually recommended when an AAA grows to >5.5 cm in diameter. While in the past the only option for the treatment of AAA was open surgery, today most are treated with Endovascular Aneurysm Repair (EVAR).[3] EVAR has been widely adopted, as EVAR has a lower risk of death associated with surgery (0.5% for EVAR vs 3% for open surgery).[4] Open surgery is sometimes still preferred to EVAR, as EVAR requires long-term surveillance with CT Scans.[5]
There is moderate evidence to support screening in individuals with risk factors for abdominal aortic aneurysms (e.g., males ≥65).
Classification
Abdominal aortic aneurysms are commonly divided according to their size and symptomatology. An aneurysm is usually defined as an outer aortic diameter over 3 cm (normal diameter of the aorta is around 2 cm).[6] If the outer diameter exceeds 5.5 cm, the aneurysm is considered to be large.[7] A ruptured AAA is a clinical diagnosis involving the presence of the triad of abdominal pain, shock and a pulsatile abdominal mass. If these conditions are present, indicating AAA rupture, no further clinical investigations are needed before surgery.[8]
Signs and symptoms
The vast majority of aneurysms are asymptomatic. However, as abdominal aortic aneurysms expand, they may become painful and lead to pulsating sensations in the abdomen or pain in the chest, lower back, or scrotum.[9] The risk of rupture is high in a symptomatic aneurysm, which is therefore considered an indication for surgery. The complications include rupture, peripheral embolization, acute aortic occlusion, and aortocaval (between the aorta and inferior vena cava) or aortoduodenal (between the aorta and theduodenum) fistulae. On physical examination, a palpable abdominal mass can be noted. Bruitscan be present in case of renal or visceral arterial stenosis.[10]
Aortic rupture
The clinical manifestation of ruptured AAA usually includes excruciating pain of the lower back, flank, abdomen and groin. The bleeding usually leads to a hypovolemic shock with hypotension,tachycardia, cyanosis, and altered mental status. The mortality of AAA rupture is up to 90%. 65–75% of patients die before they arrive at hospital and up to 90% die before they reach the operating room.[11] The bleeding can be retroperitoneal or intraperitoneal, or the rupture can create an aortocaval or aortointestinal (between the aorta and intestine) fistula.[12] Flank ecchymosis (appearance of a bruise) is a sign of retroperitoneal hemorrhage, and is also called Grey Turner's sign.[10][13]
Causes
The exact causes of the degenerative process remain unclear. There are, however, some hypotheses and well-defined risk factors.[14]
- Tobacco smoking: Greater than 90% of people who develop a AAA have smoked at some point in their life.[15]
- Alcohol and hypertension: The inflammation caused by prolonged use of alcohol and hypertensive effects from abdominal edema which leads to hemorrhoids, esophageal varices, and other conditions, is also considered a long-term cause of AAA.
- Genetic influences: The influence of genetic factors is highly probable. The high familial prevalence rate is most notable in male individuals.[16] There are many hypotheses about the exact genetic disorder that could cause higher incidence of AAA among male members of the affected families. Some presumed that the influence of alpha 1-antitrypsin deficiency could be crucial, while other experimental works favored the hypothesis of X-linked mutation, which would explain the lower incidence in heterozygous females. Other hypotheses of genetic etiology have also been formulated.[10] Connective tissue disorders, such as Marfan syndrome andEhlers-Danlos syndrome, have also been strongly associated with AAA.[12] Both relapsing polychondritis and pseudoxanthoma elasticum may cause abdominal aortic aneurysm.[17]
- Atherosclerosis: The AAA was long considered to be caused by atherosclerosis, because the walls of the AAA are frequently affected heavily. However, this hypothesis cannot be used to explain the initial defect and the development of occlusion, which is observed in the process.[10]
- Other causes: Other causes of the development of AAA include: infection, trauma, arteritis, cystic medial necrosis (m. Erdheim).[12]
Pathophysiology
The most striking histopathological changes of aneurysmatic aorta are seen in tunica media and intima. These include accumulation of lipids in foam cells, extracellular free cholesterol crystals, calcifications, thrombosis, and ulcerations and ruptures of the layers. There is anadventitial inflammatory infiltrate.[12] However, the degradation of tunica media by means of proteolytic process seems to be the basicpathophysiologic mechanism of the AAA development. Some researchers report increased expression and activity of matrixmetalloproteinases in individuals with AAA. This leads to elimination of elastin from the media, rendering the aortic wall more susceptible to the influence of the blood pressure.[10] There is also a reduced amount of vasa vasorum in the abdominal aorta (compared to the thoracic aorta); consequently, the tunica media must rely mostly on diffusion for nutrition which makes it more susceptible to damage.[18]
Hemodynamics affect the development of AAA. It has a predilection for the infrarenal aorta. The histological structure and mechanical characteristics of infrarenal aorta differ from those of the thoracic aorta. The diameter decreases from the root to the bifurcation, and the wall of the abdominal aorta also contains a lesser proportion of elastin. The mechanical tension in abdominal aortic wall is therefore higher than in the thoracic aortic wall. The elasticity and distensibility also decline with age, which can result in gradual dilatation of the segment. Higher intraluminal pressure in patients with arterial hypertension markedly contributes to the progression of the pathological process.[12] Suitable hemodynamics conditions may be linked to specific Intraluminal Thrombus (ILT) patterns along the aortic lumen, which in turn may affect AAA's development.[19]
Diagnosis
An abdominal aortic aneurysm is usually diagnosed by physical exam, ultrasound, or CT. Plain abdominal radiographs may show the outline of an aneurysm when its walls are calcified. However, this is the case in less than half of all aneurysms. Ultrasonography is used to screen for aneurysms and to determine the size of any present. Additionally, free peritoneal fluid can be detected. It is noninvasive and sensitive, but the presence of bowel gas or obesity may limit its usefulness. CT scan has a nearly 100% sensitivity for aneurysm and is also useful in preoperative planning, detailing the anatomy and possibility for endovascular repair. In the case of suspected rupture, it can also reliably detect retroperitoneal fluid. Alternative less often used methods for visualization of the aneurysm include MRI andangiography.
An aneurysm ruptures if the mechanical stress (tension per area) exceeds the local wall strength; consequently, peak wall stress (PWS)[20] and peak wall rupture risk (PWRR) [21] have been found to be more reliable parameters than diameter to assess AAA rupture risk. Medical software allows computing these rupture risk indices from standard clinical CT data and provides a patient-specific AAA rupture risk diagnosis.[22] This type of biomechanical approach has been shown to accurately predict the location of AAA rupture.[23]
Prevention
- Treatment of hypertension
- Smoking cessation
- Low-fat diet
- Screening abdominal ultrasound for men older than 65 years has been shown to reduce mortality, although cost-effectiveness has not been shown.
Screening
The U.S. Preventive Services Task Force recommends a single screening ultrasound for abdominal aortic aneurysm in males age 65 to 75 years who have a history of smoking.[24] There is an estimated number needed to screen of approximately 850 people.[25] It is unclear if screening is useful in women aged 65 to 75 who have smoked and they recommend against screening in women who have never smoked.[24]
Repeat ultrasounds should be carried out in those who have an aortic size greater than 3.0 cm.[26] In those whose aorta is between 3.0 and 3.9 cm this should be every three years, if between 4.0 and 4.4 cm every two year, and if between 4.5 and 5.4 cm every year.[26]
Management and Treatment
The treatment options for asymptomatic AAA are conservative management, surveillance with a view to eventual repair, and immediate repair. There are currently two modes of repair available for an AAA: open aneurysm repair (OR), and endovascular aneurysm repair (EVAR). An intervention is often recommended if the aneurysm grows more than 1 cm per year or it is bigger than 5.5 cm.[15] Repair is also indicated for symptomatic aneurysms.[27]
Conservative
Conservative management is indicated in patients where repair carries a high risk of mortality and in patients where repair is unlikely to improve life expectancy. The mainstay of the conservative treatment is smoking cessation.
Surveillance is indicated in small asymptomatic aneurysms (less than 5.5 cm) where the risk of repair exceeds the risk of rupture. As an AAA grows in diameter the risk of rupture increases. Surveillance until the aneurysm has reached a diameter of 5.5 cm has not been shown to have a higher risk as compared to early intervention.[28][29]
Medication
No medical therapy has been found to be effective at decreasing the growth rate or rupture rate of asymptomatic AAAs.[27] Blood pressure and lipids should however be treated like in any atherosclerotic condition.[6] Studies have suggested possible protective effects of therapy with angiotensin converting enzyme inhibitors,[30] beta-blockers,[6] and statins.[31]
Surgery
Surgery for an abdominal aortic aneurysm is known as AAA surgery or AAA repair. The threshold for repair varies slightly from individual to individual, depending on the balance of risks and benefits when considering repair versus ongoing surveillance. The size of an individual's native aorta may influence this, along with the presence of comorbidities that increase operative risk or decrease life expectancy.[27]
- Open repair
Main article: Open aortic surgery
Open repair is indicated in young patients as an elective procedure, or in growing or large, symptomatic or ruptured aneurysms. It was the main surgical intervention used from the 1950s until other procedures developed.
For most operations, the surgeon tries to use as small an incision as reasonably possible, but that is not the case for open AAA surgery because there is an overriding concern. The aorta must be clamped off during the repair, and that denies blood to the entire abdomen and both legs; this can cause a whole range of complications if the aorta is shut off too long. It is essential to make the critical part of the operation fast, so the incision is typically made as large as possible, from just below the breastbone to just above the pubic bone.
Recovery after open AAA surgery takes significant time. The minimums are a few days in intensive care, a week total time in hospital and a few months before full recovery.
- Endovascular repair
Main article: Endovascular aneurysm repair
Endovascular repair first became practical in the 1990s and although it is now an established alternative to open repair, its role is yet to be clearly defined. It is generally indicated in older, high-risk patients or patients unfit for open repair. However, endovascular repair is feasible for only a proportion of AAAs, depending on the morphology of the aneurysm. The main advantages over open repair are that there is less peri-operative mortality, less time in intensive care, less time in hospital overall and earlier return to normal activity. Disadvantages of endovascular repair include a requirement for more frequent ongoing hospital reviews, and a higher chance of further procedures being required. According to the latest studies, the EVAR procedure does not offer any benefit for overall survival or health-related quality of life compared to open surgery, although aneurysm-related mortality is lower.[32][33][34][35] In patients unfit for open repair, EVAR plus conservative management was associated with no benefit, more complications, subsequent procedures and higher costs compared to conservative management alone.[36] Endovascular treatment for paraanastomotic aneurysms after aortobiiliac reconstruction is also a possibility.[37]
A major cause of complications in EVAR is the failure of the seal between the proximal, infra-renal aneurysm neck and the endovascular graft.[38][39][40] Risk of this form of failure is especially elevated in adverse or challenging proximal neck anatomies, where this seal could be compromised by unsuitable geometric fit between the graft and vessel wall, as well as instability of the anatomy.[41][42][43] New recent techniques have been introduced to address these risks by utilizing a segment of the supra-renal portion of the aorta to increase the sealing zone, such as with fenestrated EVAR, chimneys and snorkels.[44] These techniques may be suitable in certain patients with qualifying factors, e.g. configuration of renal arteries, renal function. However, these are more complex procedures than standard EVAR and may be subject to further complications.[45][46][47]
An approach that directly augments the fixation and sealing between the graft and aorta to mimic the stability of a surgical anastomosis is EndoAnchoring.[48][49] EndoAnchors are small, helically shaped implants that directly lock the graft to the aortic wall with the goal to prevent complications of the seal, especially in adverse neck anatomies.[50][51] These EndoAnchors may also be used to treat identified leaks between the graft and proximal neck.[52][53][54] To ensure successful implantation, placement of EndoAnchors should be limited to the areas of the aortic neck free from excessive thrombus, calcification and/or plaque.
Prognosis
| AAA Size (cm) | Growth rate (cm/yr)[55] | Annual rupture risk (%)[56] |
|---|---|---|
| 3.0-3.9 | 0.39 | 0 |
| 4.0-4.9 | 0.36 | 0.5-5 |
| 5.0-5.9 | 0.43 | 3-15 |
| 6.0-6.9 | 0.64 | 10-20 |
| >=7.0 | - | 20-50 |
Although the current standard of determining rupture risk is based on maximum diameter, it is known that smaller AAAs that fall below this threshold (diameter<5.5 cm) may also rupture, and larger AAAs (diameter>5.5 cm) may remain stable.[57][58] In one report, it was shown that 10–24% of ruptured AAAs were less than 5 cm in diameter.[58] It has also been reported that of 473 non-repaired AAAs examined from autopsy reports, there were 118 cases of rupture, 13% of which were less than 5 cm in diameter. This study also showed that 60% of the AAAs greater than 5 cm (including 54% of those AAAs between 7.1 and 10 cm) never experienced rupture.[59] Vorp et al. later deduced from the findings of Darling et al. that if the maximum diameter criterion were followed for the 473 subjects, only 7% (34/473) of cases would have succumbed to rupture prior to surgical intervention as the diameter was less than 5 cm, with 25% (116/473) of cases possibly undergoing unnecessary surgery since these AAAs may never have ruptured.[59]
Alternative methods of rupture assessment have been recently reported. The majority of these approaches involve the numerical analysis of AAAs using the common engineering technique of the finite element method (FEM) to determine the wall stress distributions. Recent reports have shown that these stress distributions have been shown to correlate to the overall geometry of the AAA rather than solely to the maximum diameter.[60][61][62] It is also known that wall stress alone does not completely govern failure as an AAA will usually rupture when the wall stress exceeds the wall strength. In light of this, rupture assessment may be more accurate if both the patient-specific wall stress is coupled together with patient-specific wall strength. A non-invasive method of determining patient-dependent wall strength was recently reported,[63] with more traditional approaches to strength determination via tensile testing performed by other researchers in the field.[64][65][66] Some of the more recently proposed AAA rupture-risk assessment methods include: AAA wall stress;[20][67][68] AAA expansion rate;[69] degree of asymmetry;[62] presence of intraluminal thrombus (ILT);[70] a rupture potential index (RPI);[71][72] a finite element analysis rupture index (FEARI);[73] biomechanical factors coupled with computer analysis;[74] growth of ILT;[75] geometrical parameters of the AAA;[76] and also a method of determining AAA growth and rupture based on mathematical models.[77][78]
The post-operative mortality for an already ruptured AAA has slowly decreased over several decades but remains higher than 40%.[8]However, if the AAA is surgically repaired before rupture, the post-operative mortality rate is substantially lower: approximately 1-6%.[79]
Epidemiology
The occurrence of AAA varies markedly by ethnicity. In the United Kingdom the rate of AAA in Caucasian men older than 65 years is about 4.7%, while in Asian men it is 0.45%.[80] It is also uncommon in individuals of African, and Hispanic heritage.[citation needed]
There are 9000 deaths yearly in the U.S. secondary to AAA rupture.[81] The frequency varies strongly between males and females. The peak incidence is among males around 70 years of age, the prevalence among males over 60 years totals 2-6%. The frequency is much higher in smokers than in non-smokers (8:1), and the risk decreases slowly after smoking cessation.[82] Other risk factors includehypertension and male sex.[12] In the U.S., the incidence of AAA is 2-4% in the adult population.[10] AAA is 4-6 times more common in male siblings of known patients, with a risk of 20-30%.[83]
Rupture of the AAA occurs in 1-3% of men aged 65 or more, the mortality is 70-95%.[7]
History
The first historical records about AAA are from Ancient Rome in the 2nd century AD, when Greek surgeon Antyllus tried to treat the AAA with proximal and distal ligature, central incision and removal of thrombotic material from the aneurysm. However, attempts to treat the AAA surgically were unsuccessful until 1923. In that year, Rudolph Matas (who also proposed the concept of endoaneurysmorrhaphy), performed the first successful aortic ligation on a human.[84] Other methods that were successful in treating the AAA included wrapping the aorta with polyethene cellophane, which induced fibrosis and restricted the growth of the aneurysm. Albert Einstein was operated on by Rudolph Nissen with use of this technique in 1949, and survived five years after the operation, though he eventually died when the aneurysm ruptured.[85] Endovascular aneurysm repair was first performed in the late 1980s and has been widely adopted in the subsequent decades. Endovascular repair was first used for treating a ruptured aneurysm in Nottingham in 1994[86]
Former presidential candidate Bob Dole had an abdominal aortic aneurysm in 2001 and was treated surgically by vascular surgeonKenneth Ouriel. The operation was successful. In 1993, country music singer Conway Twitty died from AAA.[87]
Society and culture
In 2001 former presidential candidate Bob Dole underwent surgery for an abdominal aortic aneurysm in which a team of surgeons led by Doctor Kenneth Ouriel inserted a stent graft:
| “ | Ouriel said that the team inserted a Y-shaped tube through an incision in Dole's leg and placed it inside the weakened portion of the aorta. The aneurysm will eventually contract around the stent, which will remain in place for the rest of Dole's life.[87] | ” |
Actor Robert Jacks, who plays Leatherface in Texas Chainsaw Massacre: The Next Generation died from an abdominal aneurysm on August 8, 2001, just one day shy of his 42nd birthday. His father also died from the same cause when Robert was a child.
Research
Risk assessment and experimental models
There have been many calls for alternative approaches to rupture-risk assessment over the past number of years, with many believing that a biomechanics-based approach may be more suitable than the current diameter approach. Numerical modelling is a valuable tool to researchers allowing approximate wall stresses to be calculated, thus revealing the rupture potential of a particular aneurysm. Experimental models are required to validate these numerical results, and provide a further insight into the biomechanical behaviour of the AAA. In vivo, AAAs exhibit a varying range of material strengths[88] from localised weak hypoxic regions[89] to much stronger regions and areas of calcifications.[90] Experimental models can now be manufactured using a novel technique involving the injection-moulding lost-wax manufacturing process to create patient-specific anatomically correct AAA replicas.[91] Work has also focused on developing more realistic material analogues to those in vivo, and recently a novel range of silicone-rubbers was created allowing the varying material properties of the AAA to be more accurately represented.[92] These rubber models can also be used in a variety of experimental testing from stress analysis using the photoelastic method[93] to deterimining whether the locations of rupture experimentally correlate with those predicted numerically.[94] New endovascular devices are being developed that are able to treat more complex and tortuous anatomies.[95]
Prevention and treatment
A recent animal study published in the journal Nature Medicine showed that removing a single protein prevents early damage in blood vessels from triggering a later-stage, frequently lethal complication of atherosclerosis. By eliminating the gene for a signaling protein called cyclophilin A (CypA) from a strain of mice, researchers were able to provide complete protection against abdominal aortic aneurysm (AAA).[96]
Other recent research, published in the American Journal of Pathology, identified Granzyme B (GZMB) (a protein-degrading enzyme) to be a potential therapeutic target in the treatment of abdominal aortic aneurysms. Specifically, elimination of this enzyme in mice models — both slowed the progression of aneurysms and improved survival.[97][98]
With the recent advancements in AAA research, coupled with the increasing collaboration between clinicians and engineers, the future research into AAA rupture-prediction and treatment appears to be in a strong position to combat what is currently ranked as the 13th leading cause of death in the US and the 10th leading cause of death in men over the age of 55 years.










There is a safe & effective Natural Herbal Medicine. For Total Cure Call +2349010754824, or email him drrealakhigbe@gmail.com For an Appointment with (Dr.) AKHIGBE contact him. Treatment with Natural Herbal Cure. For:Dengue Fever, Malaria. Painful or Irregular Menstruation. HIV/Aids. Diabetics. Vaginal Infections. Vaginal Discharge. Itching Of the Private Part. Breast Infection. Discharge from Breast. Breast Pain & Itching. Lower Abdominal Pain. No Periods or Periods Suddenly Stop. Women Sexual Problems. High Blood Pressure Chronic Disease. Pain during Sex inside the Pelvis. Pain during Urination. Pelvic Inflammatory Disease, (PID). Dripping Of Sperm from the Vagina As Well As for Low sperm count. Parkinson disease. Obesity, Lupus. Cancer. Tuberculosis. Zero sperm count. Bacteria, Impotence Fertility,Protoplasmic, Diarrhea. Hepatitis A&B, Rabies. Asthma. Quick Ejaculation. Gallstone, Cystic Fibrosis, Schizophrenia, Crubs, Cirrhosis, Premature Ejaculation. Herpes. Joint Pain. Stroke. Cornelia Disease, Weak Erection. Ovarian problem, Erysipelas, Thyroid, Relapsing polychondritis, Discharge from Penis. Bronchial Problem, HPV. Hepatitis A and B. STD. Smallpox, Staphylococcus + Gonorrhea + Syphilis. Heart Disease. Pile-Hemorrhoid.rheumatism, Impotence, thyroid, Autism, Sepsis Bacteria, Penis enlargement, Prostate Problem, Waist & Back Pain. Male Infertility and Female Infertility. Etc. Take Action Now. contact him & Order for your Natural Herbal Medicine: +2349010754824 and email him drrealakhigbe@gmail.com Note For an Appointment with (Dr.) AKHIGBE.I suffered in Cancer for a year and three months dying in pain and full of heartbreak. One day I was searching through the internet and I came across a testimony of herpes cure by doctor Akhigbe. So I contacted him to try my luck, we talked and he sent me the medicine through courier service and with instructions on how to be drinking it.To my greatest surprise drinking the herbal medicine within three weeks I got the changes and I was cured totally. I don't really know how it happens but there is power in Dr Akhigbe herbal medicine. He is a good herbalist doctor.
ReplyDelete