Schistosomiasis
| Schistosomiasis | |
|---|---|
| Classification and external resources | |
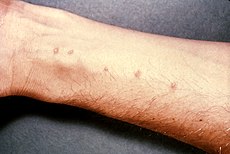
Skin blisters on the forearm, created by the entrance of Schistosoma parasite.
|
|
| ICD-10 | B65 |
| ICD-9 | 120 |
| MedlinePlus | 001321 |
| MeSH | D012552 |
The disease is spread by contact with water that contains the parasites. These parasites are released from freshwater snails that have been infected. The disease is especially common among children in developing countries as they are more likely to play in infected water. Other high risk groups include farmers, fishermen, and people using infected water for their daily chores. Diagnosis is by finding the eggs of the parasite in a person's urine or stool. It can also be confirmed by finding antibodies against the disease in the blood.[3]
Methods to prevent the disease include improving access to clean water and reducing the number of snails. In areas where the disease is common entire groups may be treated all at once and yearly with the medication praziquantel. This is done to decrease the number of people infected and therefore decrease the spread of the disease. Praziquantel is also the treatment recommended by the World Health Organization for those who are known to be infected.[3]
Schistosomiasis affects almost 210 million people worldwide,[4] and an estimated 12,000[5] to 200,000 people die from it a year.[6] The disease is most commonly found in Africa, Asia and South America.[3] Around 700 million people, in more than 70 countries, live in areas where the disease is common.[6][7] Schistosomiasis is second only to malaria, as a parasitic disease with the greatest economic impact.[8]
Classification
Species of Schistosoma that can infect humans:- Schistosoma mansoni (ICD-10 B65.1) and Schistosoma intercalatum (B65.8) cause intestinal schistosomiasis
- Schistosoma haematobium (B65.0) causes urinary schistosomiasis
- Schistosoma japonicum (B65.2) and Schistosoma mekongi (B65.8) cause Asian intestinal schistosomiasis
Species of Schistosoma that can infect other animals:
S. bovis — normally infects cattle, sheep and goats in Africa, parts of Southern Europe and the Middle East
S. mattheei — normally infects cattle, sheep and goats in Central and Southern Africa
S. margrebowiei — normally infects antelope, buffalo and waterbuck in Southern and Central Africa
S. curassoni — normally infects domestic ruminants in West Africa
S. rodhaini — normally infects rodents and carnivores in parts of Central Africa
Signs and symptoms
Above all, schistosomiasis is a chronic disease. Many infections are subclinically symptomatic, with mild anemia and malnutrition being common in endemic areas. Acute schistosomiasis (Katayama's fever) may occur weeks after the initial infection, especially by S. mansoni and S. japonicum. Manifestations include:- Abdominal pain
- Cough
- Diarrhea
- Eosinophilia — extremely high eosinophil granulocyte (white blood cell) count.
- Fever
- Fatigue
- Hepatosplenomegaly — the enlargement of both the liver and the spleen.
- Hepatic schistosomiasis is the second most common cause of esophageal varices[9] worldwide.
- Genital sores — lesions that increase vulnerability to HIV infection. Lesions caused by schistosomiasis may continue to be a problem after control of the schistosomiasis infection itself. Early treatment, especially of children, which is relatively inexpensive, prevents formation of the sores.[10][11]
- Skin symptoms: At the start of infection, mild itching and a papular dermatitis of the feet and other parts after swimming in polluted streams containing cercariae.[12]:432
- Colonic polyposis with bloody diarrhea (Schistosoma mansoni mostly);
- Portal hypertension with hematemesis and splenomegaly (S. mansoni, S. japonicum);
- Cystitis and ureteritis (S. haematobium) with hematuria, which can progress to bladder cancer;
- Pulmonary hypertension (S. mansoni, S. japonicum, more rarely S. haematobium);
- Glomerulonephritis;
- and central nervous system lesions.
Pathophysiology
Life cycle
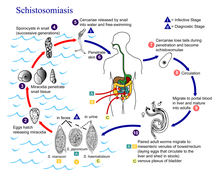
Schistosomes have a typical trematode vertebrate-invertebrate lifecycle, with humans being the definitive host.Snails
The life cycles of all five human schistosomes are broadly similar: parasite eggs are released into the environment from infected individuals, hatching on contact with fresh water to release the free-swimming miracidium. Miracidia infect freshwater snails by penetrating the snail's foot. After infection, close to the site of penetration, the miracidium transforms into a primary (mother) sporocyst. Germ cells within the primary sporocyst will then begin dividing to produce secondary (daughter) sporocysts, which migrate to the snail's hepatopancreas. Once at the hepatopancreas, germ cells within the secondary sporocyst begin to divide again, this time producing thousands of new parasites, known as cercariae, which are the larvae capable of infecting mammals.Cercariae emerge daily from the snail host in a circadian rhythm, dependent on ambient temperature and light. Young cercariae are highly mobile, alternating between vigorous upward movement and sinking to maintain their position in the water. Cercarial activity is particularly stimulated by water turbulence, by shadows and by chemicals found on human skin.
The most common way of getting schistosomiasis in developing countries is by wading or swimming in lakes, ponds and other bodies of water that are infested with the snails (usually of the genera Biomphalaria, Bulinus, or Oncomelania) that are the natural reservoirs of the Schistosoma pathogen.
Humans
Penetration of the human skin occurs after the cercaria have attached to and explored the skin. The parasite secretes enzymes that break down the skin's protein to enable penetration of the cercarial head through the skin. As the cercaria penetrates the skin it transforms into a migrating schistosomulum stage.Parasites reach maturity in six to eight weeks, at which time they begin to produce eggs. Adult S. mansoni pairs residing in the mesenteric vessels may produce up to 300 eggs per day during their reproductive lives. S. japonicum may produce up to 3,000 eggs per day. Many of the eggs pass through the walls of the blood vessels, and through the intestinal wall, to be passed out of the body in feces. S. haematobium eggs pass through the ureteral or bladder wall and into the urine. Only mature eggs are capable of crossing into the digestive tract, possibly through the release of proteolytic enzymes, but also as a function of host immune response, which fosters local tissue ulceration. Up to half the eggs released by the worm pairs become trapped in the mesenteric veins, or will be washed back into the liver, where they will become lodged. Worm pairs can live in the body for an average of four and a half years, but may persist up to twenty years.
Trapped eggs mature normally, secreting antigens that elicit a vigorous immune response. The eggs themselves do not damage the body. Rather it is the cellular infiltration resultant from the immune response that causes the pathology classically associated with schistosomiasis.
Diagnosis
| This section does not cite any references or sources. (April 2014) |
Eggs can be present in the stool in infections with all Schistosoma species. The examination can be performed on a simple smear (1 to 2 mg of fecal material). Since eggs may be passed intermittently or in small amounts, their detection will be enhanced by repeated examinations and/or concentration procedures (such as the formalin-ethyl acetate technique). In addition, for field surveys and investigational purposes, the egg output can be quantified by using the Kato technique (20 to 50 mg of fecal material) or the Ritchie technique.
Eggs can be found in the urine in infections with S. japonicum and with S. intercalatum (recommended time for collection: between noon and 3 p.m.) Detection will be enhanced by centrifugation and examination of the sediment. Quantification is possible by using filtration through a nucleopore membrane of a standard volume of urine followed by egg counts on the membrane. Investigation of S. haematobium should also include a pelvic x-ray as bladder wall calcification is highly characteristic of chronic infection.
Recently a field evaluation of a novel handheld microscope was undertaken in Uganda for the diagnosis of intestinal schistosomiasis by a team led by Russell Stothard from the Natural History Museum of London, working with the Schistosomiasis Control Initiative, London.[14]
Tissue biopsy (rectal biopsy for all species and biopsy of the bladder for S. haematobium) may demonstrate eggs when stool or urine examinations are negative.
The eggs of S. haematobium are ellipsoidal with a terminal spine, S. mansoni eggs are also ellipsoidal but with a lateral spine, S. japonicum eggs are spheroidal with a small knob.
Antibody detection can be useful in both clinical management and for epidemiologic surveys.
Prevention
A few countries have eradicated the disease, and many more are working toward it.[citation needed] The World Health Organization is promoting these efforts. In some cases, urbanization, pollution, and/or consequent destruction of snail habitat has reduced exposure, with a subsequent decrease in new infections.Snails
Prevention is best accomplished by eliminating the water-dwelling snails that are the natural reservoir of the disease. Acrolein, copper sulfate, and niclosamide can be used for this purpose. Recent studies have suggested that snail populations can be controlled by the introduction of, or augmentation of existing, crayfish populations.For many years from the 1950s onwards, vast dams and irrigation schemes were constructed, causing a massive rise in water-borne infections from schistosomiasis. The detailed specifications laid out in various UN documents since the 1950s could have minimized this problem. Irrigation schemes can be designed to make it hard for the snails to colonize the water, and to reduce the contact with the local population.[15]
This has been cited as a classic case of the relevance paradox because guidelines on how to design these schemes to minimise the spread of the disease had been published years before, but the designers were unaware of them.[16]
Treatment
Main article: Schistosomicide
Schistosomiasis is readily treated using a single oral dose of the drug praziquantel annually.[17] As with other major parasitic diseases, there is ongoing and extensive research into developing a schistosomiasis vaccine that will prevent the parasite from completing its life cycle in humans. In 2009, Eurogentec Biologics developed a vaccine against bilharziosis in partnership with INSERM and researchers from the Pasteur Institute.[18][19][20]The World Health Organization has developed guidelines for community treatment of schistosomiasis based on the impact the disease has on children in endemic villages:[17]
- When a village reports more than 50 percent of children have blood in their urine, everyone in the village receives treatment.[17]
- When 20 to 50 percent of children have bloody urine, only school-age children are treated.[17]
- When fewer than 20 percent of children have symptoms, mass treatment is not implemented.[17]
Antimony has been used in the past to treat the disease. In low doses, this toxic metalloid bonds to sulfur atoms in enzymes used by the parasite and kills it without harming the host. This treatment is not referred to in present-day peer review scholarship; praziquantel is universally used. Outside of the U.S., there is a drug available exclusively for treating Schistosoma mansoni (oxamniquine) and one exclusively for treating S. hematobium (metrifonate). While metrifonate has been discontinued for use by the British National Health Service, a Cochrane review found it equally effective in treating urinary schistosomiasis as the leading drug, praziquantel.[21]
Mirazid, an Egyptian drug made from myrrh, was under investigation for oral treatment of the disease up until 2005.[22] The efficacy of praziquantel was proven to be about eight times than that of Mirazid and therefore Mirazid was not recommended as a suitable agent to control schistosomiasis.[23]
Another agent, mefloquine, which has previously been used to treat malaria, was recognised in 2008-2009 to be effective against schistosoma.[24] Mefloquine may be used in combination with praziquantel or artemisinins. Its mechanism of action is not known but it causes extensive and severe morphological, histopathological, and ultrastructural damage to adult and juvenile schistosomes, particularly, the worm tegument, musculature, gut, and vitelline glands of female worms.
Epidemiology
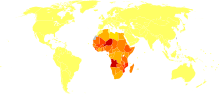
Disability-adjusted life year for schistosomiasis per 100,000 inhabitants.
no data
less than 50
50-75
75-100
100-150
150-200
200-250
250-300
300-350
350-400
400-450
450-500
more than 500
Worldwide an estimated 12,000[5] to 200,000 people die related to schistosomiasis yearly.[6]
Schistosoma mansoni is found in parts of South America and the Caribbean, Africa, and the Middle East; S. haematobium in Africa and the Middle East; and S. japonicum in the Far East. S. mekongi and S. intercalatum are found locally in Southeast Asia and central West Africa, respectively.
Among human parasitic diseases, schistosomiasis (sometimes called bilharziasis) ranks second behind malaria in terms of socio-economic and public health importance in tropical and subtropical areas. The disease is endemic in 74-76 developing countries. They live in rural agricultural and peri-urban areas.[26]
20 million have severe consequences from the disease.[27] In many areas, schistosomiasis infects a large proportion of children under 14 years of age.
History
Schistosomiasis is known as bilharzia or bilharziosis in many countries, after German physician Theodor Bilharz, who first described the cause of urinary schistosomiasis in 1851.The first doctor who described the entire disease cycle was Brazilian parasitologist Pirajá da Silva in 1908. The first known case of infection was discovered in 2014, it belongs to a child who lived 6,200 years ago.[28]
It was a common cause of death for Ancient Egyptians in the Greco-Roman Period.[29]












No comments:
Post a Comment